Cellular, pharmacological, and biophysical evaluation of explanted lungs from a patient with sickle cell disease and severe pulmonary arterial hypertension
- PMID: 25006410
- PMCID: PMC4070844
- DOI: 10.1086/674754
Cellular, pharmacological, and biophysical evaluation of explanted lungs from a patient with sickle cell disease and severe pulmonary arterial hypertension
Abstract
Pulmonary hypertension is recognized as a leading cause of morbidity and mortality in patients with sickle cell disease (SCD). We now report benchtop phenotyping from the explanted lungs of the first successful lung transplant in SCD. Pulmonary artery smooth muscle cells (PASMCs) cultured from the explanted lungs were analyzed for proliferate capacity, superoxide (O2 (•-)) production, and changes in key pulmonary arterial hypertension (PAH)-associated molecules and compared with non-PAH PASMCs. Upregulation of several pathologic processes persisted in culture in SCD lung PASMCs in spite of cell passage. SCD lung PASMCs showed growth factor- and serum-independent proliferation, upregulation of matrix genes, and increased O2 (•-) production compared with control cells. Histologic analysis of SCD-associated PAH arteries demonstrated increased and ectopically located extracellular matrix deposition and degradation of elastin fibers. Biomechanical analysis of these vessels confirmed increased arterial stiffening and loss of elasticity. Functional analysis of distal fifth-order pulmonary arteries from these lungs demonstrated increased vasoconstriction to an α1-adrenergic receptor agonist and concurrent loss of both endothelial-dependent and endothelial-independent vasodilation compared with normal pulmonary arteries. This is the first study to evaluate the molecular, cellular, functional, and mechanical changes in end-stage SCD-associated PAH.
Keywords: CD47; endothelin 1; lung transplant; matrix; pulmonary arterial hypertension; sickle cell disease; superoxide; thrombospondin 1.
Figures

 ± SD (from 3 experiments). For TSP1 messenger RNA (mRNA), single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, double asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL, the single pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1 and CTRL-Hx, and the double pound sign indicates a statistically significant difference (P < 0.05) compared with SCD-Nx, SCD-TSP1, and SCD-Hx; for CD47 mRNA, asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx; for ET-1 mRNA, single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, and double asterisks indicate a statistically significant difference (P < 0.05) compared with SCD-Nx; and for EDNRA mRNA, the single asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-Nx, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1, and double asterisks indicate a statistically significant difference (P < 0.05) compared with SCD-Nx and SCD-TSP1.
± SD (from 3 experiments). For TSP1 messenger RNA (mRNA), single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, double asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL, the single pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1 and CTRL-Hx, and the double pound sign indicates a statistically significant difference (P < 0.05) compared with SCD-Nx, SCD-TSP1, and SCD-Hx; for CD47 mRNA, asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx; for ET-1 mRNA, single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, and double asterisks indicate a statistically significant difference (P < 0.05) compared with SCD-Nx; and for EDNRA mRNA, the single asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-Nx, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1, and double asterisks indicate a statistically significant difference (P < 0.05) compared with SCD-Nx and SCD-TSP1.

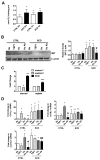
 ± SD (from 3 experiments). For collagen Iα1 (Col Iα1) messenger RNA (mRNA), single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, and double asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1; for collagen IIIα1 (Col IIIα1) mRNA, the single asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-Nx and CTRL-TSP1, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-Hx, and double asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, CTRL-TSP1, and CTRL-Hx; and for collagen IVα1 (Col IVα1) mRNA, single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx and CTRL-TSP1, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-Hx, and the double pound sign indicates a statistically significant difference (P < 0.05) compared with SCD-Nx and SCD-TSP1.
± SD (from 3 experiments). For collagen Iα1 (Col Iα1) messenger RNA (mRNA), single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, and double asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-TSP1; for collagen IIIα1 (Col IIIα1) mRNA, the single asterisk indicates a statistically significant difference (P < 0.05) compared with CTRL-Nx and CTRL-TSP1, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-Hx, and double asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx, CTRL-TSP1, and CTRL-Hx; and for collagen IVα1 (Col IVα1) mRNA, single asterisks indicate a statistically significant difference (P < 0.05) compared with CTRL-Nx and CTRL-TSP1, the pound sign indicates a statistically significant difference (P < 0.05) compared with CTRL-Hx, and the double pound sign indicates a statistically significant difference (P < 0.05) compared with SCD-Nx and SCD-TSP1.
Similar articles
-
Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice.Am J Physiol Lung Cell Mol Physiol. 2019 Jun 1;316(6):L1150-L1164. doi: 10.1152/ajplung.00302.2018. Epub 2019 Mar 20. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30892078 Free PMC article.
-
Involvement of the bone morphogenetic protein system in endothelin- and aldosterone-induced cell proliferation of pulmonary arterial smooth muscle cells isolated from human patients with pulmonary arterial hypertension.Hypertens Res. 2010 May;33(5):435-45. doi: 10.1038/hr.2010.16. Epub 2010 Feb 26. Hypertens Res. 2010. PMID: 20186146
-
Endothelin-1 induces lysyl oxidase expression in pulmonary artery smooth muscle cells.Can J Physiol Pharmacol. 2020 Sep;98(9):629-636. doi: 10.1139/cjpp-2019-0658. Epub 2020 Jul 2. Can J Physiol Pharmacol. 2020. PMID: 32615041
-
Identification of Celastramycin as a Novel Therapeutic Agent for Pulmonary Arterial Hypertension.Circ Res. 2019 Jul 19;125(3):309-327. doi: 10.1161/CIRCRESAHA.119.315229. Epub 2019 Jun 14. Circ Res. 2019. PMID: 31195886
-
[Enhanced Ca2+-sensing receptor function in pulmonary hypertension].Yakugaku Zasshi. 2013;133(12):1351-9. doi: 10.1248/yakushi.13-00228-3. Yakugaku Zasshi. 2013. PMID: 24292183 Review. Japanese.
Cited by
-
Ex Vivo Regional Mechanical Characterization of Porcine Pulmonary Arteries.Exp Mech. 2021 Jan;61(1):285-303. doi: 10.1007/s11340-020-00678-2. Epub 2021 Jan 7. Exp Mech. 2021. PMID: 33814554 Free PMC article.
-
A Novel Protective Role for Matrix Metalloproteinase-8 in the Pulmonary Vasculature.Am J Respir Crit Care Med. 2021 Dec 15;204(12):1433-1451. doi: 10.1164/rccm.202108-1863OC. Am J Respir Crit Care Med. 2021. PMID: 34550870 Free PMC article.
-
Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer.Antioxid Redox Signal. 2017 Oct 20;27(12):874-911. doi: 10.1089/ars.2017.7140. Epub 2017 Sep 8. Antioxid Redox Signal. 2017. PMID: 28712304 Free PMC article.
-
CD47 and thrombospondin-1 regulation of mitochondria, metabolism, and diabetes.Am J Physiol Cell Physiol. 2021 Aug 1;321(2):C201-C213. doi: 10.1152/ajpcell.00175.2021. Epub 2021 Jun 9. Am J Physiol Cell Physiol. 2021. PMID: 34106789 Free PMC article. Review.
-
Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease.Matrix Biol. 2014 Jul;37:92-101. doi: 10.1016/j.matbio.2014.01.002. Epub 2014 Jan 11. Matrix Biol. 2014. PMID: 24418252 Free PMC article. Review.
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031. - PubMed
-
- Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med 2000;342:1855–1865. - PubMed
-
- Abbott KC, Hypolite IO, Agodoa LY. Sickle cell nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol 2002;58:9–15. - PubMed
-
- Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am 2005;19:929–941, viii. - PubMed
-
- Mehta SH, Adams RJ. Treatment and prevention of stroke in children with sickle cell disease. Curr Treat Options Neurol 2006;8:503–512. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

