NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways
- PMID: 25006578
- PMCID: PMC4055427
- DOI: 10.1155/2014/547187
NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways
Abstract
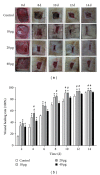
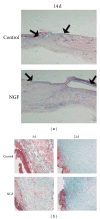
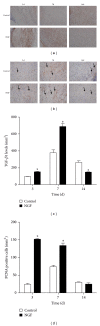
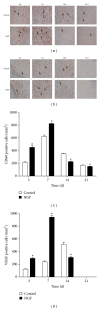
As a well-known neurotrophic factor, nerve growth factor (NGF) has also been extensively recognized for its acceleration of healing in cutaneous wounds in both animal models and randomized clinical trials. However, the underlying mechanisms accounting for the therapeutic effect of NGF on skin wounds are not fully understood. NGF treatment significantly accelerated the rate of wound healing by promoting wound reepithelialization, the formation of granulation tissue, and collagen production. To explore the possible mechanisms of this process, the expression levels of CD68, VEGF, PCNA, and TGF-β1 in wounds were detected by immunohistochemical staining. The levels of these proteins were all significantly raised in NGF-treated wounds compared to untreated controls. NGF also significantly promoted the migration, but not the proliferation, of dermal fibroblasts. NGF induced a remarkable increase in the activity of PI3K/Akt, JNK, ERK, and Rac1, and blockade with their specific inhibitors significantly impaired the NGF-induced migration. In conclusion, NGF significantly accelerated the healing of skin excisional wounds in rats and the fibroblast migration induced by NGF may contribute to this healing process. The activation of PI3K/Akt, Rac1, JNK, and ERK were all involved in the regulation of NGF-induced fibroblast migration.
Figures

Similar articles
-
The activation of the NF-κB-JNK pathway is independent of the PI3K-Rac1-JNK pathway involved in the bFGF-regulated human fibroblast cell migration.J Dermatol Sci. 2016 Apr;82(1):28-37. doi: 10.1016/j.jdermsci.2016.01.003. Epub 2016 Jan 7. J Dermatol Sci. 2016. PMID: 26829882
-
bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing.PLoS One. 2010 Aug 17;5(8):e12228. doi: 10.1371/journal.pone.0012228. PLoS One. 2010. PMID: 20808927 Free PMC article.
-
bFGF Promotes the Migration of Human Dermal Fibroblasts under Diabetic Conditions through Reactive Oxygen Species Production via the PI3K/Akt-Rac1- JNK Pathways.Int J Biol Sci. 2015 Jun 1;11(7):845-59. doi: 10.7150/ijbs.11921. eCollection 2015. Int J Biol Sci. 2015. PMID: 26078726 Free PMC article.
-
Nerve growth factor and burn wound healing: Update of molecular interactions with skin cells.Burns. 2023 Aug;49(5):989-1002. doi: 10.1016/j.burns.2022.11.001. Epub 2022 Nov 7. Burns. 2023. PMID: 36379825 Review.
-
A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms.Clin Rev Allergy Immunol. 2020 Jun;58(3):298-312. doi: 10.1007/s12016-019-08729-w. Clin Rev Allergy Immunol. 2020. PMID: 30729428 Review.
Cited by
-
Tetrahedral framework nucleic acids promote scarless healing of cutaneous wounds via the AKT-signaling pathway.Signal Transduct Target Ther. 2020 Jul 17;5(1):120. doi: 10.1038/s41392-020-0173-3. Signal Transduct Target Ther. 2020. PMID: 32678073 Free PMC article.
-
The peroxidase PRDX1 inhibits the activated phenotype in mammary fibroblasts through regulating c-Jun N-terminal kinases.BMC Cancer. 2019 Aug 16;19(1):812. doi: 10.1186/s12885-019-6031-4. BMC Cancer. 2019. PMID: 31419957 Free PMC article.
-
Neuronal PAS Domain 2 (Npas2)-Deficient Fibroblasts Accelerate Skin Wound Healing and Dermal Collagen Reconstruction.Anat Rec (Hoboken). 2020 Jun;303(6):1630-1641. doi: 10.1002/ar.24109. Epub 2019 Mar 27. Anat Rec (Hoboken). 2020. PMID: 30851151 Free PMC article.
-
Comparative proteomics of paired vocal fold and oral mucosa fibroblasts.J Proteomics. 2017 Feb 23;155:11-21. doi: 10.1016/j.jprot.2017.01.010. Epub 2017 Jan 15. J Proteomics. 2017. PMID: 28099887 Free PMC article.
-
NOX2-Dependent Reactive Oxygen Species Regulate Formyl-Peptide Receptor 1-Mediated TrkA Transactivation in SH-SY5Y Cells.Oxid Med Cell Longev. 2019 Dec 2;2019:2051235. doi: 10.1155/2019/2051235. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31871542 Free PMC article.
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. - PubMed
-
- Singer AJ, Clark RAF. Cutaneous wound healing. The New England Journal of Medicine. 1999;341(10):738–746. - PubMed
-
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clinics in Dermatology. 2007;25(1):9–18. - PubMed
-
- Braund R, Hook S, Medlicott NJ. The role of topical growth factors in chronic wounds. Current Drug Delivery. 2007;4(3):195–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

