Immunogenicity studies of bivalent inactivated virions of EV71/CVA16 formulated with submicron emulsion systems
- PMID: 25006583
- PMCID: PMC4071850
- DOI: 10.1155/2014/670506
Immunogenicity studies of bivalent inactivated virions of EV71/CVA16 formulated with submicron emulsion systems
Abstract
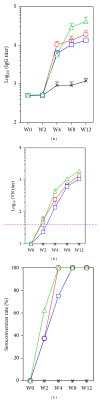
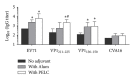
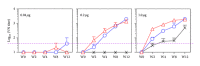
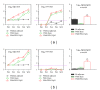
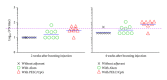
We assessed two strategies for preparing candidate vaccines against hand, foot, and mouth disease (HFMD) caused mainly by infections of enterovirus (EV) 71 and coxsackievirus (CV) A16. We firstly design and optimize the potency of adjuvant combinations of emulsion-based delivery systems, using EV71 candidate vaccine as a model. We then perform immunogenicity studies in mice of EV71/CVA16 antigen combinations formulated with PELC/CpG. A single dose of inactivated EV71 virion (0.2 μg) emulsified in submicron particles was found (i) to induce potent antigen-specific neutralizing antibody responses and (ii) consistently to elicit broad antibody responses against EV71 neutralization epitopes. A single dose immunogenicity study of bivalent activated EV71/CVA16 virion formulated with either Alum or PELC/CpG adjuvant showed that CVA16 antigen failed to elicit CVA16 neutralizing antibody responses and did not affect EV71-specific neutralizing antibody responses. A boosting dose of emulsified EV71/CVA16 bivalent vaccine candidate was found to be necessary to achieve high seroconversion of CVA16-specific neutralizing antibody responses. The current results are important for the design and development of prophylactic vaccines against HFMD and other emerging infectious diseases.
Figures
References
-
- World Health Organization. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD) 2011.
-
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. The Lancet Neurology. 2010;9(11):1097–1105. - PubMed
-
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. The Lancet Infectious Diseases. 2010;10(11):778–790. - PubMed
-
- Cheng A, Fung C-P, Liu C-C, et al. A phase I, randomized, open-label study to evaluate the safety and immunogenicity of an enterovirus 71 vaccine. Vaccine. 2013;31(20):2471–2476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

