Synthesis of riboflavines, quinoxalinones and benzodiazepines through chemoselective flow based hydrogenations
- PMID: 25006783
- PMCID: PMC6271593
- DOI: 10.3390/molecules19079736
Synthesis of riboflavines, quinoxalinones and benzodiazepines through chemoselective flow based hydrogenations
Abstract
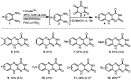
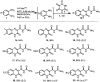
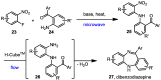
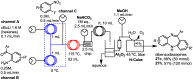
Robust chemical routes towards valuable bioactive entities such as riboflavines, quinoxalinones and benzodiazepines are described. These make use of modern flow hydrogenation protocols enabling the chemoselective reduction of nitro group containing building blocks in order to rapidly generate the desired amine intermediates in situ. In order to exploit the benefits of continuous processing the individual steps were transformed into a telescoped flow process delivering selected benzodiazepine products on scales of 50 mmol and 120 mmol respectively.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Multisite solid catalyst for cascade reactions: the direct synthesis of benzodiazepines from nitro compounds.Chemistry. 2009 Sep 7;15(35):8834-41. doi: 10.1002/chem.200900492. Chemistry. 2009. PMID: 19621393
-
Stereoselective Synthesis of Benzo[e][1,4]oxazino[4,3-a][1,4]diazepine-6,12-diones with Two Diversity Positions.ACS Comb Sci. 2017 Dec 11;19(12):770-774. doi: 10.1021/acscombsci.7b00134. Epub 2017 Nov 2. ACS Comb Sci. 2017. PMID: 29048877
-
Iridium catalyzed asymmetric hydrogenation of cyclic imines of benzodiazepinones and benzodiazepines.Org Lett. 2012 Aug 3;14(15):3890-3. doi: 10.1021/ol301620z. Epub 2012 Jul 17. Org Lett. 2012. PMID: 22799531
-
Recent advances in synthesis and medicinal chemistry of benzodiazepines.Bioorg Chem. 2020 Apr;97:103668. doi: 10.1016/j.bioorg.2020.103668. Epub 2020 Feb 17. Bioorg Chem. 2020. PMID: 32106040 Review.
-
The expanding utility of continuous flow hydrogenation.Org Biomol Chem. 2015 Jul 14;13(26):7119-30. doi: 10.1039/c5ob01067e. Epub 2015 Jun 15. Org Biomol Chem. 2015. PMID: 26073166 Review.
Cited by
-
Hepatoprotective Principles and Other Chemical Constituents from the Mycelium of Phellinus linteus.Molecules. 2018 Jul 12;23(7):1705. doi: 10.3390/molecules23071705. Molecules. 2018. PMID: 30002357 Free PMC article.
-
The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry.Beilstein J Org Chem. 2015 Jul 17;11:1194-219. doi: 10.3762/bjoc.11.134. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26425178 Free PMC article. Review.
References
-
- Silverman R.B. The Organic Chemistry of Drug Design and Drug Action. 2nd ed. Academic Press; San Diego, CA, USA: 2004.
-
- Li J.-J., Johnson D.S., Sliskovich D.R., Roth B.D. Contemporary Drug Synthesis. John Wiley and Sons; Chichester, UK: 2004.
-
- Wermuth C.G. The Practice of Medicinal Chemistry. 3rd ed. Elsevier Academic Press; Burlington, MA, USA: 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

