Synthesis of riboflavines, quinoxalinones and benzodiazepines through chemoselective flow based hydrogenations
- PMID: 25006783
- PMCID: PMC6271593
- DOI: 10.3390/molecules19079736
Synthesis of riboflavines, quinoxalinones and benzodiazepines through chemoselective flow based hydrogenations
Abstract
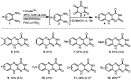
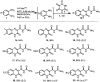
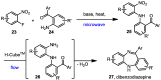
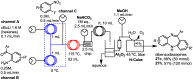
Robust chemical routes towards valuable bioactive entities such as riboflavines, quinoxalinones and benzodiazepines are described. These make use of modern flow hydrogenation protocols enabling the chemoselective reduction of nitro group containing building blocks in order to rapidly generate the desired amine intermediates in situ. In order to exploit the benefits of continuous processing the individual steps were transformed into a telescoped flow process delivering selected benzodiazepine products on scales of 50 mmol and 120 mmol respectively.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Silverman R.B. The Organic Chemistry of Drug Design and Drug Action. 2nd ed. Academic Press; San Diego, CA, USA: 2004.
-
- Li J.-J., Johnson D.S., Sliskovich D.R., Roth B.D. Contemporary Drug Synthesis. John Wiley and Sons; Chichester, UK: 2004.
-
- Wermuth C.G. The Practice of Medicinal Chemistry. 3rd ed. Elsevier Academic Press; Burlington, MA, USA: 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

