Cell-cell adhesions and cell contractility are upregulated upon desmosome disruption
- PMID: 25006807
- PMCID: PMC4090201
- DOI: 10.1371/journal.pone.0101824
Cell-cell adhesions and cell contractility are upregulated upon desmosome disruption
Abstract
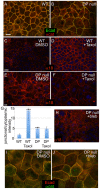
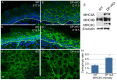
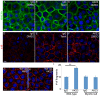
Desmosomes are perturbed in a number of disease states - including genetic disorders, autoimmune and bacterial diseases. Here, we report unexpected changes in other cell-cell adhesion structures upon loss of desmosome function. We found that perturbation of desmosomes by either loss of the core desmosomal protein desmoplakin or treatment with pathogenic anti-desmoglein 3 (Dsg3) antibodies resulted in changes in adherens junctions consistent with increased tension. The total amount of myosin IIA was increased in desmoplakin-null epidermis, and myosin IIA became highly localized to cell contacts in both desmoplakin-null and anti-Dsg3-treated mouse keratinocytes. Inhibition of myosin II activity reversed the changes to adherens junctions seen upon desmosome disruption. The increased cortical myosin IIA promoted epithelial sheet fragility, as myosin IIA-null cells were less susceptible to disruption by anti-Dsg3 antibodies. In addition to the changes in adherens junctions, we found a significant increase in the expression of a number of claudin genes, which encode for transmembrane components of the tight junction that provide barrier function. These data demonstrate that desmosome disruption results in extensive transcriptional and posttranslational changes that alter the activity of other cell adhesion structures.
Conflict of interest statement
Figures

Similar articles
-
Evidence for the Desmosomal Cadherin Desmoglein-3 in Regulating YAP and Phospho-YAP in Keratinocyte Responses to Mechanical Forces.Int J Mol Sci. 2019 Dec 10;20(24):6221. doi: 10.3390/ijms20246221. Int J Mol Sci. 2019. PMID: 31835537 Free PMC article.
-
Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous Dsg3.J Invest Dermatol. 2011 Mar;131(3):706-18. doi: 10.1038/jid.2010.389. Epub 2010 Dec 16. J Invest Dermatol. 2011. PMID: 21160493 Free PMC article.
-
E-cadherin and Src associate with extradesmosomal Dsg3 and modulate desmosome assembly and adhesion.Cell Mol Life Sci. 2015 Dec;72(24):4885-97. doi: 10.1007/s00018-015-1977-0. Epub 2015 Jun 27. Cell Mol Life Sci. 2015. PMID: 26115704 Free PMC article.
-
Desmosomal adhesion and pemphigus vulgaris: the first half of the story.Cell Commun Adhes. 2013 Feb;20(1-2):1-10. doi: 10.3109/15419061.2013.763799. Epub 2013 Feb 1. Cell Commun Adhes. 2013. PMID: 23368972 Review.
-
150(th) anniversary series: Desmosomes and autoimmune disease, perspective of dynamic desmosome remodeling and its impairments in pemphigus.Cell Commun Adhes. 2014 Dec;21(6):269-80. doi: 10.3109/15419061.2014.943397. Epub 2014 Jul 31. Cell Commun Adhes. 2014. PMID: 25078507 Review.
Cited by
-
Modulation of Mechanical Stress Mitigates Anti-Dsg3 Antibody-Induced Dissociation of Cell-Cell Adhesion.Adv Biol (Weinh). 2021 Jan;5(1):e2000159. doi: 10.1002/adbi.202000159. Epub 2021 Jan 4. Adv Biol (Weinh). 2021. PMID: 33724731 Free PMC article.
-
Cell adhesion in epidermal development and barrier formation.Curr Top Dev Biol. 2015;112:383-414. doi: 10.1016/bs.ctdb.2014.11.027. Epub 2015 Feb 11. Curr Top Dev Biol. 2015. PMID: 25733147 Free PMC article. Review.
-
The Modulatory Influence of Plant-Derived Compounds on Human Keratinocyte Function.Int J Mol Sci. 2021 Nov 19;22(22):12488. doi: 10.3390/ijms222212488. Int J Mol Sci. 2021. PMID: 34830374 Free PMC article. Review.
-
Cardiac Biomarkers and Autoantibodies in Endurance Athletes: Potential Similarities with Arrhythmogenic Cardiomyopathy Pathogenic Mechanisms.Int J Mol Sci. 2021 Jun 17;22(12):6500. doi: 10.3390/ijms22126500. Int J Mol Sci. 2021. PMID: 34204386 Free PMC article. Review.
-
Critical Role of Hepatic Cyp450s in the Testis-Specific Toxicity of (5R)-5-Hydroxytriptolide in C57BL/6 Mice.Front Pharmacol. 2017 Nov 21;8:832. doi: 10.3389/fphar.2017.00832. eCollection 2017. Front Pharmacol. 2017. PMID: 29209210 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

