Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment
- PMID: 25007089
- PMCID: PMC4253029
- DOI: 10.1055/s-0034-1376889
Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment
Abstract
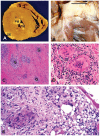
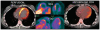
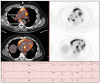
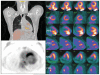
Clinically evident sarcoidosis involving the heart has been noted in at least 2 to 7% of patients with sarcoidosis, but occult involvement is much higher (> 20%). Cardiac sarcoidosis is often not recognized antemortem, as sudden death may be the presenting feature. Cardiac involvement may occur at any point during the course of sarcoidosis and may occur in the absence of pulmonary or systemic involvement. Sarcoidosis can involve any part of the heart, with protean manifestations. Prognosis of cardiac sarcoidosis is related to extent and site(s) of involvement. Most deaths due to cardiac sarcoidosis are due to arrhythmias or conduction defects, but granulomatous infiltration of the myocardium may be lethal. The definitive diagnosis of isolated cardiac sarcoidosis is difficult. The yield of endomyocardial biopsies is low; treatment of cardiac sarcoidosis is often warranted even in the absence of histologic proof. Radionuclide scans are integral to the diagnosis. Currently, 18F-fluorodeoxyglucose positron emission tomography/computed tomography and gadolinium-enhanced magnetic resonance imaging scans are the key imaging modalities to diagnose cardiac sarcoidosis. The prognosis of cardiac sarcoidosis is variable, but mortality rates of untreated cardiac sarcoidosis are high. Although randomized therapeutic trials have not been done, corticosteroids (alone or combined with additional immunosuppressive medications) remain the mainstay of treatment. Because of the potential for sudden cardiac death, implantable cardioverter-defibrillators should be placed in any patient with cardiac sarcoidosis and serious ventricular arrhythmias or heart block, and should be considered for cardiomyopathy. Cardiac transplantation is a viable option for patients with end-stage cardiac sarcoidosis refractory to medical therapy.
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Figures

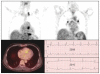
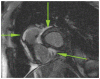
Similar articles
-
Cardiac Involvement in Sarcoidosis: Evolving Concepts in Diagnosis and Treatment.Semin Respir Crit Care Med. 2017 Aug;38(4):477-498. doi: 10.1055/s-0037-1602381. Epub 2017 Jul 27. Semin Respir Crit Care Med. 2017. PMID: 28750462 Review. No abstract available.
-
Cardiac sarcoidosis.Semin Respir Crit Care Med. 2010 Aug;31(4):428-41. doi: 10.1055/s-0030-1262211. Epub 2010 Jul 27. Semin Respir Crit Care Med. 2010. PMID: 20665393 Review.
-
Cardiac manifestations of sarcoidosis and therapeutic options.Cardiol Rev. 2009 Jul-Aug;17(4):153-8. doi: 10.1097/CRD.0b013e3181a1f763. Cardiol Rev. 2009. PMID: 19525676 Review.
-
Cardiac manifestations of sarcoidosis: diagnosis and management.Eur Heart J. 2017 Sep 14;38(35):2663-2670. doi: 10.1093/eurheartj/ehw328. Eur Heart J. 2017. PMID: 27469375
-
Cardiac magnetic resonance imaging-indeterminate/negative cardiac sarcoidosis revealed by 18F-fluorodeoxyglucose-positron emission tomography: two case reports and a review of the literature.J Med Case Rep. 2017 Oct 20;11(1):291. doi: 10.1186/s13256-017-1453-6. J Med Case Rep. 2017. PMID: 29052526 Free PMC article. Review.
Cited by
-
The chameleon of cardiology: cardiac sarcoidosis before and after heart transplantation.ESC Heart Fail. 2020 Apr;7(2):692-696. doi: 10.1002/ehf2.12581. Epub 2019 Dec 5. ESC Heart Fail. 2020. PMID: 31802644 Free PMC article.
-
Lyme disease and cardiac sarcoidosis: Management of associated ventricular arrhythmias.HeartRhythm Case Rep. 2018 Sep 11;4(12):584-588. doi: 10.1016/j.hrcr.2018.09.001. eCollection 2018 Dec. HeartRhythm Case Rep. 2018. PMID: 30581738 Free PMC article. No abstract available.
-
Atrial tachyarrhythmia as a presenting symptom leading to the diagnosis of pulmonary sarcoidosis treated with catheter-based ablation.Proc (Bayl Univ Med Cent). 2021 Jul 23;34(6):712-714. doi: 10.1080/08998280.2021.1945363. eCollection 2021. Proc (Bayl Univ Med Cent). 2021. PMID: 34732998 Free PMC article.
-
Sarcoidosis and the heart: A review of the literature.Intractable Rare Dis Res. 2015 Nov;4(4):170-80. doi: 10.5582/irdr.2015.01023. Intractable Rare Dis Res. 2015. PMID: 26668777 Free PMC article. Review.
-
Screening for cardiac sarcoidosis: diagnostic approach and long-term follow-up in a tertiary centre.Neth Heart J. 2025 Feb;33(2):55-64. doi: 10.1007/s12471-024-01925-0. Epub 2025 Jan 9. Neth Heart J. 2025. PMID: 39786690 Free PMC article.
References
-
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. - PubMed
-
- Fleming H. Cardiac sarcoidosis. In: James DG, editor. Sarcoidosis and other granulomatous disorders. Marcel Dekker; New York: 1994. pp. 323–334.
-
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999 Am J Respir Crit Care Med. 1999;160(2):736–755. - PubMed
-
- Johns CJ, Michele TM. The clinical management of sarcoidosis. A 50-year experience at the Johns Hopkins Hospital. Medicine (Baltimore) 1999;78(2):65–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

