Direct evidence of iron uptake by the Gram-positive siderophore-shuttle mechanism without iron reduction
- PMID: 25007174
- PMCID: PMC4168784
- DOI: 10.1021/cb500319n
Direct evidence of iron uptake by the Gram-positive siderophore-shuttle mechanism without iron reduction
Abstract
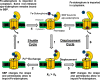
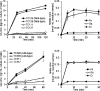
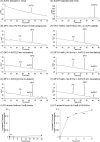
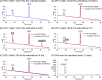
Iron is an essential element for all organisms, and microorganisms produce small molecule iron-chelators, siderophores, to efficiently acquire Fe(III). Gram-positive bacteria possess lipoprotein siderophore-binding proteins (SBPs) on the membrane. Some of the SBPs bind both apo-siderophores (iron-free) and Fe-siderophore (iron-chelated) and only import Fe-siderophores. When the SBP initially binds an apo-siderophore, the SBP uses the Gram-positive siderophore-shuttle mechanism (the SBPs exchange Fe(III) from a Fe-siderophore to the apo-siderophore bound to the protein) and/or displacement mechanism (the apo-siderophore bound to the SBP is released and a Fe-siderophore is then bound to the protein) to import the Fe-siderophore. Previously, we reported that the Bacillus cereus SBP, YxeB, exchanges Fe(III) from a ferrioxamine B (FO) to a desferrioxamine B (DFO) bound to YxeB using the siderophore-shuttle mechanism although the iron exchange was indirectly elucidated. Synthetic Cr-DFO (inert metal FO analog) and Ga-DFO (nonreducible FO analog) are bound to YxeB and imported via YxeB and the corresponding permeases and ATPase. YxeB exchanges Fe(III) from FO and Ga(III) from Ga-DFO to DFO bound to the protein, indicating that the metal-exchange occurs without metal reduction. YxeB also binds DFO derivatives including acetylated DFO (apo-siderophore) and acetylated FO (AcFO, Fe-siderophore). The iron from AcFO is transferred to DFO when bound to YxeB, giving direct evidence of iron exchange. Moreover, YxeB also uses the displacement mechanism when ferrichrome (Fch) is added to the DFO:YxeB complex. Uptake by the displacement mechanism is a minor pathway compared to the shuttle mechanism.
Figures

Similar articles
-
Gram-positive siderophore-shuttle with iron-exchange from Fe-siderophore to apo-siderophore by Bacillus cereus YxeB.Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):13821-6. doi: 10.1073/pnas.1304235110. Epub 2013 Aug 7. Proc Natl Acad Sci U S A. 2013. PMID: 23924612 Free PMC article.
-
Siderophore-mediated iron acquisition systems in Bacillus cereus: Identification of receptors for anthrax virulence-associated petrobactin.Biochemistry. 2009 Apr 28;48(16):3645-57. doi: 10.1021/bi8018674. Biochemistry. 2009. PMID: 19254027 Free PMC article.
-
Pseudomonas aeruginosa FpvB Is a High-Affinity Transporter for Xenosiderophores Ferrichrome and Ferrioxamine B.mBio. 2023 Feb 28;14(1):e0314922. doi: 10.1128/mbio.03149-22. Epub 2022 Dec 12. mBio. 2023. PMID: 36507834 Free PMC article.
-
Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways.Amino Acids. 2013 May;44(5):1267-77. doi: 10.1007/s00726-013-1468-2. Epub 2013 Feb 27. Amino Acids. 2013. PMID: 23443998 Review.
-
Iron uptake by fungi: contrasted mechanisms with internal or external reduction.Adv Microb Physiol. 2000;43:39-74. doi: 10.1016/s0065-2911(00)43002-x. Adv Microb Physiol. 2000. PMID: 10907554 Review.
Cited by
-
Transcriptome profile of Corynebacterium pseudotuberculosis in response to iron limitation.BMC Genomics. 2019 Aug 20;20(1):663. doi: 10.1186/s12864-019-6018-1. BMC Genomics. 2019. PMID: 31429699 Free PMC article.
-
Targeting Iron - Respiratory Reciprocity Promotes Bacterial Death.bioRxiv [Preprint]. 2024 Mar 1:2024.03.01.582947. doi: 10.1101/2024.03.01.582947. bioRxiv. 2024. Update in: J Biol Chem. 2025 May;301(5):108455. doi: 10.1016/j.jbc.2025.108455. PMID: 38464199 Free PMC article. Updated. Preprint.
-
Grab 'n Go: Siderophore-Binding Proteins Provide Pathogens a Quick Fix to Satisfy Their Hunger for Iron.ACS Cent Sci. 2020 Apr 22;6(4):456-458. doi: 10.1021/acscentsci.0c00179. Epub 2020 Mar 17. ACS Cent Sci. 2020. PMID: 32341992 Free PMC article. No abstract available.
-
Genome-Wide Search for Genes Required for Bifidobacterial Growth under Iron-Limitation.Front Microbiol. 2017 May 31;8:964. doi: 10.3389/fmicb.2017.00964. eCollection 2017. Front Microbiol. 2017. PMID: 28620359 Free PMC article.
-
Metal chelation as an antibacterial strategy for Pseudomonas aeruginosa and Acinetobacter baumannii.RSC Chem Biol. 2024 Sep 24;5(11):1083-96. doi: 10.1039/d4cb00175c. Online ahead of print. RSC Chem Biol. 2024. PMID: 39372678 Free PMC article. Review.
References
-
- Byers B. R., and Arceneaux J. E. L. (1998) Metal Ions in Biological Systems (Sigel A., and Sigel H., Eds) Vol. 35, pp 37–66, Marcel Dekker, Inc., New York. - PubMed
-
- Boukhalfa H.; Crumbliss A. L. (2002) Chemical aspects of siderophore mediated iron transport. Biometals 15, 325–339. - PubMed
-
- Braun V., Hantke K., and Koster W. (1998) Metal Ions in Biological Systems (Sigel A., and Sigel H., Eds.) Vol. 35, pp 67–145, Marcel Dekker, Inc., New York. - PubMed
-
- Singh A.; Severance S.; Kaur N.; Wiltsie W.; Kosman D. J. (2006) Assembly, activation, and trafficking of the Fet3p.Ftr1p high affinity iron permease complex in Saccharomyces cerevisiae. J. Biol. Chem. 281, 13355–13364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

