Pulmonary permeability assessed by fluorescent-labeled dextran instilled intranasally into mice with LPS-induced acute lung injury
- PMID: 25007191
- PMCID: PMC4090205
- DOI: 10.1371/journal.pone.0101925
Pulmonary permeability assessed by fluorescent-labeled dextran instilled intranasally into mice with LPS-induced acute lung injury
Abstract
Background: Several different methods have been used to assess pulmonary permeability in response to acute lung injury (ALI). However, these methods often involve complicated procedures and algorithms that are difficult to precisely control. The purpose of the current study is to establish a feasible method to evaluate alterations in lung permeability by instilling fluorescently labeled dextran (FITC-Dextran) intranasally.
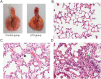
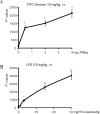
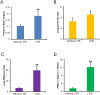
Methods/principal findings: For the mouse model of direct ALI, lipopolysaccharide (LPS) was administered intranasally. FITC-Dextran was instilled intranasally one hour before the mice were euthanized. Plasma fluorescence intensities from the LPS group were significantly higher than in the control group. To determine the reliability and reproducibility of the procedure, we also measured the lung wet-to-dry weight ratio, the protein concentration of the bronchoalveolar lavage fluid, tight and adherens junction markers and pathological changes. Consistent results were observed when the LPS group was compared with the control group. Simultaneously, we found that the concentration of plasma FITC-Dextran was LPS dose-dependent. The concentration of plasma FITC-Dextran also increased with initial intranasal FITC-Dextran doses. Furthermore, increased fluorescence intensity of plasma FITC-Dextran was found in the intraperitoneally LPS-induced ALI model.
Conclusion/significance: In conclusion, the measurement of FITC-Dextran in plasma after intranasal instillation is a simple, reliable, and reproducible method to evaluate lung permeability alterations in vivo. The concentration of FITC-Dextran in the plasma may be useful as a potential peripheral biomarker of ALI in experimental clinical studies.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

