The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy
- PMID: 25007327
- PMCID: PMC4206534
- DOI: 10.4161/auto.29329
The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy
Abstract
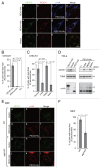
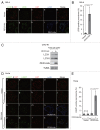
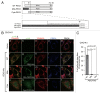
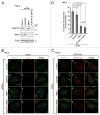
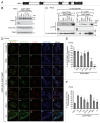
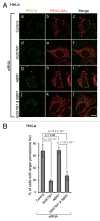
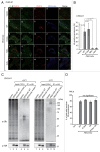
Peroxisomes are degraded by a selective type of autophagy known as pexophagy. Several different types of pexophagy have been reported in mammalian cells. However, the mechanisms underlying how peroxisomes are recognized by autophagy-related machinery remain elusive. PEX3 is a peroxisomal membrane protein (PMP) that functions in the import of PMPs into the peroxisomal membrane and has been shown to interact with pexophagic receptor proteins during pexophagy in yeast. Thus, PEX3 is important not only for peroxisome biogenesis, but also for peroxisome degradation. However, whether PEX3 is involved in the degradation of peroxisomes in mammalian cells is unclear. Here, we report that high levels of PEX3 expression induce pexophagy. In PEX3-loaded cells, peroxisomes are ubiquitinated, clustered, and degraded in lysosomes. Peroxisome targeting of PEX3 is essential for the initial step of this degradation pathway. The degradation of peroxisomes is inhibited by treatment with autophagy inhibitors or siRNA against NBR1, which encodes an autophagic receptor protein. These results indicate that ubiquitin- and NBR1-mediated pexophagy is induced by increased expression of PEX3 in mammalian cells. In addition, another autophagic receptor protein, SQSTM1/p62, is required only for the clustering of peroxisomes. Expression of a PEX3 mutant with substitution of all lysine and cysteine residues by arginine and alanine, respectively, also induces peroxisome ubiquitination and degradation, hence suggesting that ubiquitination of PEX3 is dispensable for pexophagy and an endogenous, unidentified peroxisomal protein is ubiquitinated on the peroxisomal membrane.
Keywords: NBR1; PEX3; SQSTM1/p62; autophagy; peroxisomal membrane protein; peroxisome; pexophagy; ubiquitin.
Figures

Similar articles
-
Pexophagy is responsible for 65% of cases of peroxisome biogenesis disorders.Autophagy. 2017 May 4;13(5):991-994. doi: 10.1080/15548627.2017.1291480. Epub 2017 Feb 28. Autophagy. 2017. PMID: 28318378 Free PMC article. Review.
-
PEX13 prevents pexophagy by regulating ubiquitinated PEX5 and peroxisomal ROS.Autophagy. 2023 Jun;19(6):1781-1802. doi: 10.1080/15548627.2022.2160566. Epub 2023 Jan 1. Autophagy. 2023. PMID: 36541703 Free PMC article.
-
Peroxisomal Pex3 activates selective autophagy of peroxisomes via interaction with the pexophagy receptor Atg30.J Biol Chem. 2015 Mar 27;290(13):8623-31. doi: 10.1074/jbc.M114.619338. Epub 2015 Feb 18. J Biol Chem. 2015. PMID: 25694426 Free PMC article.
-
The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway.Autophagy. 2020 Jan;16(1):154-166. doi: 10.1080/15548627.2019.1603546. Epub 2019 Apr 21. Autophagy. 2020. PMID: 31007124 Free PMC article.
-
Mammalian pexophagy at a glance.J Cell Sci. 2024 May 1;137(9):jcs259775. doi: 10.1242/jcs.259775. Epub 2024 May 16. J Cell Sci. 2024. PMID: 38752931 Free PMC article. Review.
Cited by
-
Pexophagy in yeast and mammals: an update on mysteries.Histochem Cell Biol. 2018 Nov;150(5):473-488. doi: 10.1007/s00418-018-1724-3. Epub 2018 Sep 21. Histochem Cell Biol. 2018. PMID: 30238155 Review.
-
Development of a Novel Autophagy-related Prognostic Signature for Serous Ovarian Cancer.J Cancer. 2018 Oct 18;9(21):4058-4071. doi: 10.7150/jca.25587. eCollection 2018. J Cancer. 2018. PMID: 30410611 Free PMC article.
-
The Peroxisome-Autophagy Redox Connection: A Double-Edged Sword?Front Cell Dev Biol. 2021 Dec 16;9:814047. doi: 10.3389/fcell.2021.814047. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977048 Free PMC article. Review.
-
Pexophagy: Molecular Mechanisms and Implications for Health and Diseases.Mol Cells. 2018 Jan 31;41(1):55-64. doi: 10.14348/molcells.2018.2245. Epub 2018 Jan 23. Mol Cells. 2018. PMID: 29370694 Free PMC article. Review.
-
Molecular definitions of autophagy and related processes.EMBO J. 2017 Jul 3;36(13):1811-1836. doi: 10.15252/embj.201796697. Epub 2017 Jun 8. EMBO J. 2017. PMID: 28596378 Free PMC article. Review.
References
-
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiol Rev. 1966;46:323–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
