REV-ERBα inhibits the PTGS2 expression in bovine uterus endometrium stromal and epithelial cells exposed to ovarian steroids
- PMID: 25007867
- PMCID: PMC4219993
- DOI: 10.1262/jrd.2014-040
REV-ERBα inhibits the PTGS2 expression in bovine uterus endometrium stromal and epithelial cells exposed to ovarian steroids
Abstract
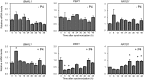
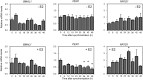
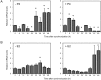
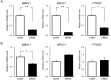
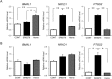
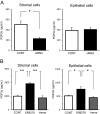
The nuclear receptor REV-ERBα (encoded by NR1D1) has a critical role in metabolism and physiology as well as circadian rhythm. Here, we investigated the possible contribution of clock genes including NR1D1 to the secretion of prostaglandin F2α (PGF2α) from bovine uterine stromal (USCs) and epithelial cells (UECs) by modulating the expression of PTGS2. The circadian oscillation of clock genes in the cells was weak compared with that reported in rodents, but the expression of BMAL1, PER1, and NR1D1 was changed temporally by treatment with ovarian steroids. Significant expression of clock genes including NR1D1 was detected in USCs exposed to progesterone. NR1D1 was also significantly expressed in UECs exposed to estradiol. The expression of PTGS2 was suppressed in USCs exposed to progesterone, while the expression was initially suppressed in UECs exposed to estradiol and then increased after long-term exposure to estradiol. BMAL1 knockdown with specific siRNA caused a significant decrease in the transcript levels of NR1D1 and PTGS2 in USCs, but not in UECs. The production of PGF2α also decreased in USCs after BMAL1 knockdown, while its level did not significantly change in UECs. The transcript level of PTGS2 was increased by treatment with the antagonist of REV-ERBα in both cell types, but the agonist was ineffective. In these two cell types treated with the agonist or antagonist, the PGF2α production coincided well with the PTGS2 expression. Collectively, these results indicate that REV-ERBα plays an inhibitory role in the expression of PTGS2 in both bovine USCs and UECs treated with ovarian steroids.
Figures

References
-
- Flint AP, Lamming GE, Stewart HJ, Abayasekara DR. The role of the endometrial oxytocin receptor in determining the length of the sterile oestrous cycle and ensuring maintenance of luteal function in early pregnancy in ruminants. Philos Trans R Soc Lond B Biol Sci 1994; 344: 291–304. - PubMed
-
- Wathes DC, Lamming GE. The oxytocin receptor, luteolysis and the maintenance of pregnancy. J Reprod Fertil Suppl 1995; 49: 53–67. - PubMed
-
- Goff AK. Steroid hormone modulation of prostaglandin secretion in the ruminant endometrium during the estrous cycle. Biol Reprod 2004; 71: 11–16. - PubMed
-
- Silvia WJ, Lewis GS, McCracken JA, Thatcher WW, Wilson L., JrHormonal regulation of uterine secretion of prostaglandin F2 alpha during luteolysis in ruminants. Biol Reprod 1991; 45: 655–663. - PubMed
-
- Jenner LJ, Parkinson TJ, Lamming GE. Uterine oxytocin receptors in cyclic and pregnant cows. J Reprod Fertil 1991; 91: 49–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

