Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics
- PMID: 25008081
- PMCID: PMC4233703
- DOI: 10.1098/rsif.2014.0526
Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics
Abstract
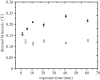
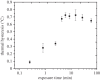
Ice-binding proteins that aid the survival of freeze-avoiding, cold-adapted organisms by inhibiting the growth of endogenous ice crystals are called antifreeze proteins (AFPs). The binding of AFPs to ice causes a separation between the melting point and the freezing point of the ice crystal (thermal hysteresis, TH). TH produced by hyperactive AFPs is an order of magnitude higher than that produced by a typical fish AFP. The basis for this difference in activity remains unclear. Here, we have compared the time dependence of TH activity for both hyperactive and moderately active AFPs using a custom-made nanolitre osmometer and a novel microfluidics system. We found that the TH activities of hyperactive AFPs were time-dependent, and that the TH activity of a moderate AFP was almost insensitive to time. Fluorescence microscopy measurement revealed that despite their higher TH activity, hyperactive AFPs from two insects (moth and beetle) took far longer to accumulate on the ice surface than did a moderately active fish AFP. An ice-binding protein from a bacterium that functions as an ice adhesin rather than as an antifreeze had intermediate TH properties. Nevertheless, the accumulation of this ice adhesion protein and the two hyperactive AFPs on the basal plane of ice is distinct and extensive, but not detectable for moderately active AFPs. Basal ice plane binding is the distinguishing feature of antifreeze hyperactivity, which is not strictly needed in fish that require only approximately 1°C of TH. Here, we found a correlation between the accumulation kinetics of the hyperactive AFP at the basal plane and the time sensitivity of the measured TH.
Keywords: antifreeze proteins; ice-binding; thermal hysteresis.
Figures





Similar articles
-
When are antifreeze proteins in solution essential for ice growth inhibition?Langmuir. 2015 Jun 2;31(21):5805-11. doi: 10.1021/acs.langmuir.5b00345. Epub 2015 May 18. Langmuir. 2015. PMID: 25946514
-
Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis.Cryobiology. 2010 Dec;61(3):327-34. doi: 10.1016/j.cryobiol.2010.10.158. Epub 2010 Oct 25. Cryobiology. 2010. PMID: 20977900
-
New insights into ice growth and melting modifications by antifreeze proteins.J R Soc Interface. 2012 Dec 7;9(77):3249-59. doi: 10.1098/rsif.2012.0388. Epub 2012 Jul 11. J R Soc Interface. 2012. PMID: 22787007 Free PMC article.
-
Antifreeze proteins enable plants to survive in freezing conditions.J Biosci. 2014 Dec;39(5):931-44. doi: 10.1007/s12038-014-9468-2. J Biosci. 2014. PMID: 25431421 Review.
-
Divergent Mechanisms of Ice Growth Inhibition by Antifreeze Proteins.Methods Mol Biol. 2024;2730:169-181. doi: 10.1007/978-1-0716-3503-2_12. Methods Mol Biol. 2024. PMID: 37943458 Review.
Cited by
-
Investigation of changes in structure and thermodynamic of spruce budworm antifreeze protein under subfreezing temperature.Sci Rep. 2017 Jan 20;7:40032. doi: 10.1038/srep40032. Sci Rep. 2017. PMID: 28106056 Free PMC article.
-
Ice-binding proteins and the applicability and limitations of the kinetic pinning model.Philos Trans A Math Phys Eng Sci. 2019 Jun 3;377(2146):20180391. doi: 10.1098/rsta.2018.0391. Philos Trans A Math Phys Eng Sci. 2019. PMID: 30982449 Free PMC article.
-
Investigating the Interaction Between Ice-Binding Proteins and Ice Surfaces Using Microfluidic Devices and Cold Stages.Methods Mol Biol. 2024;2730:109-119. doi: 10.1007/978-1-0716-3503-2_8. Methods Mol Biol. 2024. PMID: 37943454
-
A brief review of applications of antifreeze proteins in cryopreservation and metabolic genetic engineering.3 Biotech. 2019 Sep;9(9):329. doi: 10.1007/s13205-019-1861-y. Epub 2019 Aug 12. 3 Biotech. 2019. PMID: 31448185 Free PMC article. Review.
-
From ice-binding proteins to bio-inspired antifreeze materials.Soft Matter. 2017 Jul 19;13(28):4808-4823. doi: 10.1039/c6sm02867e. Soft Matter. 2017. PMID: 28657626 Free PMC article. Review.
References
-
- Janech MG, Krell A, Mock T, Kang JS, Raymond JA. 2006. Ice-binding proteins from sea ice diatoms (Bacillariophyceae). J. Phycol. 42, 410–416. (10.1111/j.1529-8817.2006.00208.x) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

