Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells
- PMID: 25008093
- PMCID: PMC4232084
- DOI: 10.1093/neuonc/nou128
Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells
Abstract
Background: Recent evidence suggests that astrocytes protect cancer cells from chemotherapy by stimulating upregulation of anti-apoptotic genes in those cells. We investigated the possibility that activation of the endothelin axis orchestrates survival gene expression and chemoprotection in MDA-MB-231 breast cancer cells and H226 lung cancer cells.
Methods: Cancer cells, murine astrocytes, and murine fibroblasts were grown in isolation, and expression of endothelin (ET) peptides and ET receptors (ETAR and ETBR) compared with expression on cancer cells and astrocytes (or cancer cells and fibroblasts) that were co-incubated for 48 hours. Type-specific endothelin receptor antagonists were used to evaluate the contribution of ETAR and ETBR to astrocyte-induced activation of the protein kinase B (AKT)/mitogen-activated protein kinase (MAPK) signal transduction pathways, anti-apoptotic gene expression, and chemoprotection of cancer cells. We also investigated the chemoprotective potential of brain endothelial cells and microglial cells.
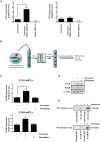
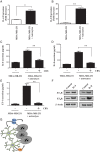
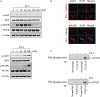
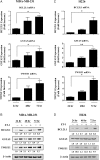
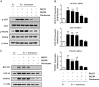
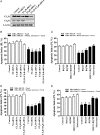
Results: Gap junction signaling between MDA-MB-231 cancer cells and astrocytes stimulates upregulation of interleukin 6 (IL-6) and IL-8 expression in cancer cells, which increases ET-1 production from astrocytes and ET receptor expression on cancer cells. ET-1 signals for activation of AKT/MAPK and upregulation of survival proteins that protect cancer cells from taxol. Brain endothelial cell-mediated chemoprotection of cancer cells also involves endothelin signaling. Dual antagonism of ETAR and ETBR is required to abolish astrocyte- and endothelial cell-mediated chemoprotection.
Conclusions: Bidirectional signaling between astrocytes and cancer cells involves upregulation and activation of the endothelin axis, which protects cancer cells from cytotoxicity induced by chemotherapeutic drugs.
Keywords: astrocytes; brain metastasis; breast cancer; endothelin; therapeutic resistance.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
The brain microenvironment: friend or foe for metastatic tumor cells?Neuro Oncol. 2014 Dec;16(12):1565-6. doi: 10.1093/neuonc/nou308. Epub 2014 Nov 13. Neuro Oncol. 2014. PMID: 25395460 Free PMC article. No abstract available.
Similar articles
-
Treatment of experimental human breast cancer and lung cancer brain metastases in mice by macitentan, a dual antagonist of endothelin receptors, combined with paclitaxel.Neuro Oncol. 2016 Apr;18(4):486-96. doi: 10.1093/neuonc/now037. Neuro Oncol. 2016. PMID: 26995790 Free PMC article.
-
Activation of ETA Receptor by Endothelin-1 Induces Hepatocellular Carcinoma Cell Migration and Invasion via ERK1/2 and AKT Signaling Pathways.J Membr Biol. 2016 Apr;249(1-2):119-28. doi: 10.1007/s00232-015-9854-1. Epub 2015 Oct 26. J Membr Biol. 2016. PMID: 26501871
-
The brain microenvironment: friend or foe for metastatic tumor cells?Neuro Oncol. 2014 Dec;16(12):1565-6. doi: 10.1093/neuonc/nou308. Epub 2014 Nov 13. Neuro Oncol. 2014. PMID: 25395460 Free PMC article. No abstract available.
-
[Astrocytes and endothelins: possibilities for tissue-repair in damaged central nervous system].Nihon Yakurigaku Zasshi. 1997 Mar;109(3):129-43. doi: 10.1254/fpj.109.129. Nihon Yakurigaku Zasshi. 1997. PMID: 9108561 Review. Japanese.
-
The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy.Can J Physiol Pharmacol. 2008 Aug;86(8):473-84. doi: 10.1139/Y08-058. Can J Physiol Pharmacol. 2008. PMID: 18758494 Review.
Cited by
-
Thorny ground, rocky soil: Tissue-specific mechanisms of tumor dormancy and relapse.Semin Cancer Biol. 2022 Jan;78:104-123. doi: 10.1016/j.semcancer.2021.05.007. Epub 2021 May 9. Semin Cancer Biol. 2022. PMID: 33979673 Free PMC article. Review.
-
[Tumor Cells and Micro-environment in Brain Metastases].Zhongguo Fei Ai Za Zhi. 2016 Sep 20;19(9):626-35. doi: 10.3779/j.issn.1009-3419.2016.09.12. Zhongguo Fei Ai Za Zhi. 2016. PMID: 27666556 Free PMC article. Review. Chinese.
-
Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: summary of a multidisciplinary roundtable discussion.ESMO Open. 2018 Jan 26;3(1):e000262. doi: 10.1136/esmoopen-2017-000262. eCollection 2018. ESMO Open. 2018. PMID: 29387475 Free PMC article. Review.
-
Mechanisms and Therapy for Cancer Metastasis to the Brain.Front Oncol. 2018 May 24;8:161. doi: 10.3389/fonc.2018.00161. eCollection 2018. Front Oncol. 2018. PMID: 29881714 Free PMC article. Review.
-
Differences in Endothelin B Receptor Isoforms Expression and Function in Breast Cancer Cells.J Cancer. 2020 Feb 19;11(9):2688-2701. doi: 10.7150/jca.41004. eCollection 2020. J Cancer. 2020. PMID: 32201539 Free PMC article.
References
-
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. - PubMed
-
- Kienast Y, Winkler F. Therapy and prophylaxis of brain metastases. Expert Rev Anticancer Ther. 2010;10(11):1763–1777. - PubMed
-
- Walbert T, Gilbert MR. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Int J Clin Oncol. 2009;14(4):299–306. - PubMed
-
- Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

