The IGF-1 receptor inhibitor picropodophyllin potentiates the anti-myeloma activity of a BH3-mimetic
- PMID: 25008202
- PMCID: PMC4294345
- DOI: 10.18632/oncotarget.1933
The IGF-1 receptor inhibitor picropodophyllin potentiates the anti-myeloma activity of a BH3-mimetic
Abstract
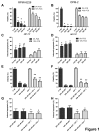
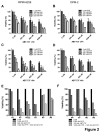
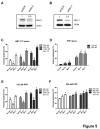
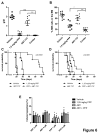
The ABT-analogous 737, 263 and 199 are BH3 mimetics showing potent anti-myeloma (MM) activity, but only on defined molecular subgroups of MM patients presenting a Bcl-2high/Mcl-1low profile. IGF-1 is a major survival factor in MM regulating the expression of Bcl-2 proteins and might therefore be a resistance factor to these ABT-analogous. We first show that IGF-1 protected human MM cell lines (HMCLs) against ABT-737. Concurrently, the IGF-1 receptor inhibitor picropodophyllin (PPP) synergistically sensitized HMCL, primary human MM and murine 5T33MM cells to ABT-737 and ABT-199 by further decreasing cell viability and enhancing apoptosis. Knockdown of Bcl-2 by shRNA protected MM cells to ABT-737, while Mcl-1 shRNA sensitized the cells. PPP overcame the Bcl-2 dependency of ABT-737, but failed to completely overcome the protective effect of Mcl-1. In vivo, co-treatment of 5T33MM bearing mice significantly decreased tumor burden and prolonged overall survival both in a prophylactic and therapeutic setting. Interestingly, proteasome inhibitor resistant CD138- 5T33MM cells were more sensitive to ABT-737, whereas PPP alone targeted the CD138+ cells more effectively. After co-treatment, both subpopulations were targeted equally. Together, the combination of an IGF-1R inhibitor and an ABT-analogue displays synergistic anti-myeloma activity providing the rational for further (pre)clinical testing.
Conflict of interest statement
Olle Larson and Magnus Axelson have ownership interest (including patents) on Owner of patent for picropodophyllin. The remaining authors declare no potential conflict of interest.
Figures


References
-
- Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nature reviews Clinical oncology. 2012;9:135–143. - PubMed
-
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. - PubMed
-
- Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Critical reviews in oncology/hematology. 2009;71(2):89–101. - PubMed
-
- Scarfo L, Ghia P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett. 2013;155:36–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

