Vesicle-associated membrane protein 2 (VAMP2) but Not VAMP3 mediates cAMP-stimulated trafficking of the renal Na+-K+-2Cl- co-transporter NKCC2 in thick ascending limbs
- PMID: 25008321
- PMCID: PMC4156046
- DOI: 10.1074/jbc.M114.589333
Vesicle-associated membrane protein 2 (VAMP2) but Not VAMP3 mediates cAMP-stimulated trafficking of the renal Na+-K+-2Cl- co-transporter NKCC2 in thick ascending limbs
Abstract
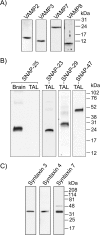
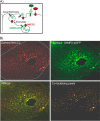
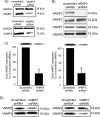
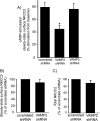
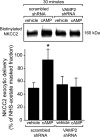
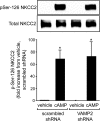
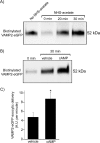
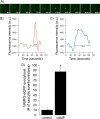
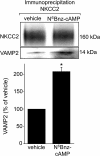
In the kidney, epithelial cells of the thick ascending limb (TAL) reabsorb NaCl via the apical Na(+)/K(+)/2Cl(-) co-transporter NKCC2. Steady-state surface NKCC2 levels in the apical membrane are maintained by a balance between exocytic delivery, endocytosis, and recycling. cAMP is the second messenger of hormones that enhance NaCl absorption. cAMP stimulates NKCC2 exocytic delivery via protein kinase A (PKA), increasing steady-state surface NKCC2. However, the molecular mechanism involved has not been studied. We found that several members of the SNARE family of membrane fusion proteins are expressed in TALs. Here we report that NKCC2 co-immunoprecipitates with VAMP2 in rat TALs, and they co-localize in discrete domains at the apical surface. cAMP stimulation enhanced VAMP2 exocytic delivery to the plasma membrane of renal cells, and stimulation of PKA enhanced VAMP2-NKCC2 co-immunoprecipitation in TALs. In vivo silencing of VAMP2 but not VAMP3 in TALs blunted cAMP-stimulated steady-state surface NKCC2 expression and completely blocked cAMP-stimulated NKCC2 exocytic delivery. VAMP2 was not involved in constitutive NKCC2 delivery. We concluded that VAMP2 but not VAMP3 selectively mediates cAMP-stimulated NKCC2 exocytic delivery and surface expression in TALs. We also demonstrated that cAMP stimulation enhances VAMP2 exocytosis and promotes VAMP2 interaction with NKCC2.
Keywords: Cyclic AMP (cAMP); Epithelial Cell; Epithelial Sodium Transport; Exocytosis; Kidney; Membrane Trafficking; Na-K-Cl Co-transporter 2 (NKCC2); SNARE Proteins.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Vesicle-associated Membrane Protein 3 (VAMP3) Mediates Constitutive Trafficking of the Renal Co-transporter NKCC2 in Thick Ascending Limbs: ROLE IN RENAL FUNCTION AND BLOOD PRESSURE.J Biol Chem. 2016 Oct 14;291(42):22063-22073. doi: 10.1074/jbc.M116.735167. Epub 2016 Aug 22. J Biol Chem. 2016. PMID: 27551042 Free PMC article.
-
cAMP stimulates apical exocytosis of the renal Na(+)-K(+)-2Cl(-) cotransporter NKCC2 in the thick ascending limb: role of protein kinase A.J Biol Chem. 2009 Sep 11;284(37):24965-71. doi: 10.1074/jbc.M109.037135. Epub 2009 Jul 10. J Biol Chem. 2009. PMID: 19592485 Free PMC article.
-
Vesicle-associated membrane protein-2 (VAMP2) mediates cAMP-stimulated renin release in mouse juxtaglomerular cells.J Biol Chem. 2011 Aug 12;286(32):28608-18. doi: 10.1074/jbc.M111.225839. Epub 2011 Jun 27. J Biol Chem. 2011. PMID: 21708949 Free PMC article.
-
Trafficking and regulation of the NKCC2 cotransporter in the thick ascending limb.Curr Opin Nephrol Hypertens. 2017 Sep;26(5):392-397. doi: 10.1097/MNH.0000000000000351. Curr Opin Nephrol Hypertens. 2017. PMID: 28614115 Review.
-
Molecular regulation of NKCC2 in the thick ascending limb.Am J Physiol Renal Physiol. 2011 Dec;301(6):F1143-59. doi: 10.1152/ajprenal.00396.2011. Epub 2011 Sep 7. Am J Physiol Renal Physiol. 2011. PMID: 21900458 Free PMC article. Review.
Cited by
-
Molecular regulation of NKCC2 in blood pressure control and hypertension.Curr Opin Nephrol Hypertens. 2019 Sep;28(5):474-480. doi: 10.1097/MNH.0000000000000531. Curr Opin Nephrol Hypertens. 2019. PMID: 31313674 Free PMC article. Review.
-
AAV-CRISPR/Cas9-Mediated Depletion of VEGFR2 Blocks Angiogenesis In Vitro.Invest Ophthalmol Vis Sci. 2017 Dec 1;58(14):6082-6090. doi: 10.1167/iovs.17-21902. Invest Ophthalmol Vis Sci. 2017. PMID: 29204648 Free PMC article.
-
Regulation of renal Na-(K)-Cl cotransporters by vasopressin.Pflugers Arch. 2017 Aug;469(7-8):889-897. doi: 10.1007/s00424-017-2002-2. Epub 2017 Jun 2. Pflugers Arch. 2017. PMID: 28577072 Review.
-
Update on NKCC2 regulation in the thick ascending limb (TAL) by membrane trafficking, phosphorylation, and protein-protein interactions.Front Physiol. 2024 Dec 9;15:1508806. doi: 10.3389/fphys.2024.1508806. eCollection 2024. Front Physiol. 2024. PMID: 39717823 Free PMC article. Review.
-
Real-time monitoring of NKCC2 endocytosis by total internal reflection fluorescence (TIRF) microscopy.Am J Physiol Renal Physiol. 2016 Jan 15;310(2):F183-91. doi: 10.1152/ajprenal.00104.2015. Epub 2015 Nov 4. Am J Physiol Renal Physiol. 2016. PMID: 26538436 Free PMC article.
References
-
- Nielsen S., Maunsbach A. B., Ecelbarger C. A., Knepper M. A. (1998) Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am. J. Physiol. 275, F885–F893 - PubMed
-
- Kaplan M. R., Plotkin M. D., Lee W. S., Xu Z. C., Lytton J., Hebert S. C. (1996) Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int. 49, 40–47 - PubMed
-
- Ortiz P. A. (2006) cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am. J. Physiol. 290, F608–F616 - PubMed
-
- Giménez I., Forbush B. (2003) Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J. Biol. Chem. 278, 26946–26951 - PubMed
-
- Meade P., Hoover R. S., Plata C., Vázquez N., Bobadilla N. A., Gamba G., Hebert S. C. (2003) cAMP-dependent activation of the renal-specific Na+-K+-2Cl− cotransporter is mediated by regulation of cotransporter trafficking. Am. J. Physiol. 284, F1145–F1154 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

