Initiation of sleep-dependent cortical-hippocampal correlations at wakefulness-sleep transition
- PMID: 25008411
- PMCID: PMC4157176
- DOI: 10.1152/jn.00783.2013
Initiation of sleep-dependent cortical-hippocampal correlations at wakefulness-sleep transition
Abstract
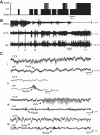
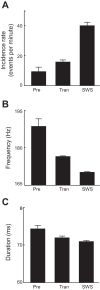
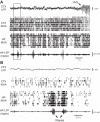
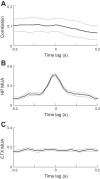
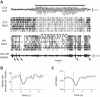
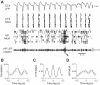
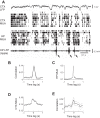
Sleep is involved in memory consolidation. Current theories propose that sleep-dependent memory consolidation requires active communication between the hippocampus and neocortex. Indeed, it is known that neuronal activities in the hippocampus and various neocortical areas are correlated during slow-wave sleep. However, transitioning from wakefulness to slow-wave sleep is a gradual process. How the hippocampal-cortical correlation is established during the wakefulness-sleep transition is unknown. By examining local field potentials and multiunit activities in the rat hippocampus and visual cortex, we show that the wakefulness-sleep transition is characterized by sharp-wave ripple events in the hippocampus and high-voltage spike-wave events in the cortex, both of which are accompanied by highly synchronized multiunit activities in the corresponding area. Hippocampal ripple events occur earlier than the cortical high-voltage spike-wave events, and hippocampal ripple incidence is attenuated by the onset of cortical high-voltage spike waves. This attenuation leads to a temporary weak correlation in the hippocampal-cortical multiunit activities, which eventually evolves to a strong correlation as the brain enters slow-wave sleep. The results suggest that the hippocampal-cortical correlation is established through a concerted, two-step state change that first synchronizes the neuronal firing within each brain area and then couples the synchronized activities between the two regions.
Keywords: high-voltage spike waves; hippocampus; memory consolidation; ripples; sleep.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
LSD degrades hippocampal spatial representations and suppresses hippocampal-visual cortical interactions.Cell Rep. 2021 Sep 14;36(11):109714. doi: 10.1016/j.celrep.2021.109714. Cell Rep. 2021. PMID: 34525364 Free PMC article.
-
Coordinated Interaction between Hippocampal Sharp-Wave Ripples and Anterior Cingulate Unit Activity.J Neurosci. 2016 Oct 12;36(41):10663-10672. doi: 10.1523/JNEUROSCI.1042-16.2016. J Neurosci. 2016. PMID: 27733616 Free PMC article.
-
Hippocampal-Prefrontal Reactivation during Learning Is Stronger in Awake Compared with Sleep States.J Neurosci. 2017 Dec 6;37(49):11789-11805. doi: 10.1523/JNEUROSCI.2291-17.2017. Epub 2017 Oct 31. J Neurosci. 2017. PMID: 29089440 Free PMC article.
-
Hippocampal ripples as a mode of communication with cortical and subcortical areas.Hippocampus. 2020 Jan;30(1):39-49. doi: 10.1002/hipo.22997. Epub 2018 Nov 13. Hippocampus. 2020. PMID: 30069976 Review.
-
Hippocampal information processing across sleep/wake cycles.Neurosci Res. 2017 May;118:30-47. doi: 10.1016/j.neures.2017.04.018. Epub 2017 May 12. Neurosci Res. 2017. PMID: 28506629 Review.
Cited by
-
Behaviourally modulated hippocampal theta oscillations in the ferret persist during both locomotion and immobility.Nat Commun. 2022 Oct 7;13(1):5905. doi: 10.1038/s41467-022-33507-2. Nat Commun. 2022. PMID: 36207304 Free PMC article.
-
Activities of visual cortical and hippocampal neurons co-fluctuate in freely moving rats during spatial behavior.Elife. 2015 Sep 8;4:e08902. doi: 10.7554/eLife.08902. Elife. 2015. PMID: 26349031 Free PMC article.
-
LSD degrades hippocampal spatial representations and suppresses hippocampal-visual cortical interactions.Cell Rep. 2021 Sep 14;36(11):109714. doi: 10.1016/j.celrep.2021.109714. Cell Rep. 2021. PMID: 34525364 Free PMC article.
-
Direct Medial Entorhinal Cortex Input to Hippocampal CA1 Is Crucial for Extended Quiet Awake Replay.Neuron. 2017 Sep 27;96(1):217-227.e4. doi: 10.1016/j.neuron.2017.09.017. Neuron. 2017. PMID: 28957670 Free PMC article.
-
Altered Cortical and Hippocampal Excitability in TgF344-AD Rats Modeling Alzheimer's Disease Pathology.Cereb Cortex. 2019 Jun 1;29(6):2716-2727. doi: 10.1093/cercor/bhy140. Cereb Cortex. 2019. PMID: 29920597 Free PMC article.
References
-
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol 73: 20–38, 1995b - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

