Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmaco-magnetoencephalography study
- PMID: 25008416
- PMCID: PMC4157173
- DOI: 10.1152/jn.00383.2014
Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmaco-magnetoencephalography study
Abstract
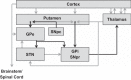
Parkinson's disease (PD) is a progressive debilitating neurodegenerative disorder clinically manifest by motor, posture and gait abnormalities. Human neurophysiological studies recording local field potentials within the subthalamic nucleus and scalp-based electroencephalography have shown pathological beta synchrony throughout the basal ganglia-thalamic-cortical motor network in PD. Notably, suppression of this pathological beta synchrony by dopamine replacement therapy or deep-brain stimulation has been associated with improved motor function. However, due to the invasive nature of these studies, it remains unknown whether this "pathological beta" is actually stronger than that observed in healthy demographically matched controls. We used magnetoencephalography to investigate neuronal synchrony and oscillatory amplitude in the beta range and lower frequencies during the resting state in patients with PD and a matched group of patients without neurological disease. Patients with PD were studied both in the practically defined drug "OFF" state, and after administration of dopamine replacements. We found that beta oscillatory amplitude was reduced bilaterally in the primary motor regions of unmedicated patients with PD compared with controls. Administration of dopaminergic medications significantly increased beta oscillatory activity, thus having a normalizing effect. Interestingly, we also found significantly stronger beta synchrony (i.e., hypersynchrony) between the primary motor regions in unmedicated patients with PD compared with controls, and that medication reduced this coupling which is in agreement with the intraoperative studies. These results are consistent with the known functionality of the basal ganglia-thalamic-cortical motor circuit and the likely consequences of beta hypersynchrony in the subthalamic nucleus of patients with PD.
Keywords: MEG; cortex; magnetoencephalography; oscillations; resting state.
Copyright © 2014 the American Physiological Society.
Figures



References
-
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol 16: 213–221, 2006 - PubMed
-
- Baudrexel S, Witte T, Seifried C, von Wegner F, Beissner F, Klein JC, Steinmetz H, Deichmann R, Roeper J, Hilker R. Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson's disease. Neuroimage 55: 1728–1738, 2011 - PubMed
-
- Bosboom JL, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, Wolters E. Resting state oscillatory brain dynamics in Parkinson's disease: an MEG study. Clin Neurophysiol 117: 2521–2531, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

