Diabetic Mesenchymal Stem Cells Are Ineffective for Improving Limb Ischemia Due to Their Impaired Angiogenic Capability
- PMID: 25008576
- PMCID: PMC4621803
- DOI: 10.3727/096368914X682792
Diabetic Mesenchymal Stem Cells Are Ineffective for Improving Limb Ischemia Due to Their Impaired Angiogenic Capability
Abstract
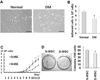
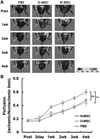
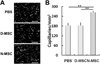
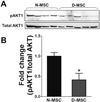
The purpose of this study was to investigate the effects of diabetes on mesenchymal stem cells (MSCs) in terms of their angiogenic and therapeutic potential for repairing tissue ischemia. We culture-isolated MSCs from streptozotocin-induced diabetic rats (D-MSCs) and compared their proliferation, differentiation, and angiogenic effects with those from normal rats (N-MSCs). The angiogenic effects of MSCs were evaluated by real-time PCR, in vitro tube formation assay, and transplantation of the MSCs into a hindlimb ischemia model followed by laser Doppler perfusion imaging. The number of MSCs derived from diabetic rats was smaller, and their proliferation rate was slower than N-MSCs. Upon induction of differentiation, the osteogenic and angiogenic differentiation of D-MSCs were aberrant compared to N-MSCs. The expression of angiogenic factors was lower in D-MSCs than N-MSCs. D-MSCs cocultured with endothelial cells resulted in decreased tube formation compared to N-MSCs. D-MSCs were ineffective to improve hindlimb ischemia and showed lower capillary density and angiogenic gene expression in ischemic limbs than N-MSCs. D-MSCs have defective proliferation and angiogenic activities and are ineffective for repairing hindlimb ischemia. Newer measures are needed before MSCs can be employed as a source for autologous cell therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K, Galipeau J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003;10(8):621–629. - PubMed
-
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102(32):11474–11479. - PMC - PubMed
-
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J. Cell. Biochem. 2003;89(6):1235–1249. - PubMed
-
- Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58(5):1229–1236. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

