The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events
- PMID: 25008921
- PMCID: PMC4178860
- DOI: 10.1128/JVI.00320-14
The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events
Abstract
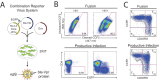
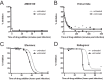
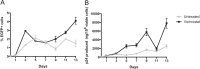
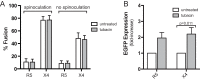
Latently infected cells remain a primary barrier to eradication of HIV-1. Over the past decade, a better understanding of the molecular mechanisms by which latency is established and maintained has led to the discovery of a number of compounds that selectively reactivate latent proviruses without inducing polyclonal T cell activation. Recently, the histone deacetylase (HDAC) inhibitor vorinostat has been demonstrated to induce HIV transcription from latently infected cells when administered to patients. While vorinostat will be given in the context of antiretroviral therapy (ART), infection of new cells by induced virus remains a clinical concern. Here, we demonstrate that vorinostat significantly increases the susceptibility of CD4(+) T cells to infection by HIV in a dose- and time-dependent manner that is independent of receptor and coreceptor usage. Vorinostat does not enhance viral fusion with cells but rather enhances the kinetics and efficiency of postentry viral events, including reverse transcription, nuclear import, and integration, and enhances viral production in a spreading-infection assay. Selective inhibition of the cytoplasmic class IIb HDAC6 with tubacin recapitulated the effect of vorinostat. These findings reveal a previously unknown cytoplasmic effect of HDAC inhibitors promoting productive infection of CD4(+) T cells that is distinct from their well-characterized effects on nuclear histone acetylation and long-terminal-repeat (LTR) transcription. Our results indicate that careful monitoring of patients and ART intensification are warranted during vorinostat treatment and indicate that HDAC inhibitors that selectively target nuclear class I HDACs could reactivate latent HIV without increasing the susceptibility of uninfected cells to HIV.
Importance: HDAC inhibitors, particularly vorinostat, are currently being investigated clinically as part of a "shock-and-kill" strategy to purge latent reservoirs of HIV. We demonstrate here that vorinostat increases the susceptibility of uninfected CD4(+) T cells to infection with HIV, raising clinical concerns that vorinostat may reseed the viral reservoirs it is meant to purge, particularly under conditions of suboptimal drug exposure. We demonstrate that vorinostat acts following viral fusion and enhances the kinetics and efficiency of reverse transcription, nuclear import, and integration. The effect of vorinostat was recapitulated using the cytoplasmic histone deacetylase 6 (HDAC6) inhibitor tubacin, revealing a novel and previously unknown cytoplasmic mechanism of HDAC inhibitors on HIV replication that is distinct from their well-characterized effects of long-terminal-repeat (LTR)-driven gene expression. Moreover, our results suggest that treatment of patients with class I-specific HDAC inhibitors could induce latent viruses without increasing the susceptibility of uninfected cells to HIV.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
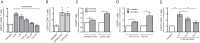

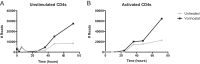
Similar articles
-
Short Communication: The Broad-Spectrum Histone Deacetylase Inhibitors Vorinostat and Panobinostat Activate Latent HIV in CD4(+) T Cells In Part Through Phosphorylation of the T-Loop of the CDK9 Subunit of P-TEFb.AIDS Res Hum Retroviruses. 2016 Feb;32(2):169-73. doi: 10.1089/AID.2015.0347. AIDS Res Hum Retroviruses. 2016. PMID: 26727990 Free PMC article.
-
Analysis of the effect of HDAC inhibitors on the formation of the HIV reservoir.mBio. 2024 Sep 11;15(9):e0163224. doi: 10.1128/mbio.01632-24. Epub 2024 Aug 13. mBio. 2024. PMID: 39136440 Free PMC article.
-
Reactivation of Latent HIV-1 Expression by Engineered TALE Transcription Factors.PLoS One. 2016 Mar 2;11(3):e0150037. doi: 10.1371/journal.pone.0150037. eCollection 2016. PLoS One. 2016. PMID: 26933881 Free PMC article.
-
Reactivation of latent HIV by histone deacetylase inhibitors.Trends Microbiol. 2013 Jun;21(6):277-85. doi: 10.1016/j.tim.2013.02.005. Epub 2013 Mar 18. Trends Microbiol. 2013. PMID: 23517573 Free PMC article. Review.
-
CXCR4 Targeting Nanoplatform for Transcriptional Activation of Latent HIV-1 Infected T Cells.ACS Appl Bio Mater. 2024 Aug 19;7(8):4831-4842. doi: 10.1021/acsabm.3c00456. Epub 2023 Aug 16. ACS Appl Bio Mater. 2024. PMID: 37586084 Review.
Cited by
-
Human Galectin-9 Is a Potent Mediator of HIV Transcription and Reactivation.PLoS Pathog. 2016 Jun 2;12(6):e1005677. doi: 10.1371/journal.ppat.1005677. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27253379 Free PMC article.
-
Current strategies to induce selective killing of HIV-1-infected cells.J Leukoc Biol. 2022 Nov;112(5):1273-1284. doi: 10.1002/JLB.4MR0422-636R. Epub 2022 Jun 16. J Leukoc Biol. 2022. PMID: 35707952 Free PMC article. Review.
-
A Lachnospiraceae-dominated bacterial signature in the fecal microbiota of HIV-infected individuals from Colombia, South America.Sci Rep. 2018 Mar 14;8(1):4479. doi: 10.1038/s41598-018-22629-7. Sci Rep. 2018. PMID: 29540734 Free PMC article.
-
Regulation of Chemokines and Cytokines by Histone Deacetylases and an Update on Histone Decetylase Inhibitors in Human Diseases.Int J Mol Sci. 2019 Mar 5;20(5):1110. doi: 10.3390/ijms20051110. Int J Mol Sci. 2019. PMID: 30841513 Free PMC article. Review.
-
Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS.AIDS. 2016 May 15;30(8):1163-74. doi: 10.1097/QAD.0000000000001079. AIDS. 2016. PMID: 26990633 Free PMC article.
References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. 10.1126/science.278.5341.1295 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

