Development of a Gaussia luciferase-based human norovirus protease reporter system: cell type-specific profile of Norwalk virus protease precursors and evaluation of inhibitors
- PMID: 25008934
- PMCID: PMC4178847
- DOI: 10.1128/JVI.01111-14
Development of a Gaussia luciferase-based human norovirus protease reporter system: cell type-specific profile of Norwalk virus protease precursors and evaluation of inhibitors
Abstract
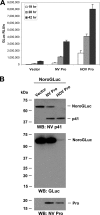
Norwalk virus (NV) is the prototype strain of human noroviruses (HuNoVs), a group of positive-strand RNA viruses in the Caliciviridae family and the leading cause of epidemic gastroenteritis worldwide. Investigation of HuNoV replication and development of antiviral therapeutics in cell culture remain challenging tasks. Here, we present NoroGLuc, a HuNoV protease reporter system based on a fusion of NV p41 protein with a naturally secreted Gaussia luciferase (GLuc), linked by the p41/p22 cleavage site for NV protease (Pro). trans cleavage of NoroGLuc by NV Pro or Pro precursors results in release and secretion of an active GLuc. Using this system, we observed a cell type-specific activity profile of NV Pro and Pro precursors, suggesting that the activity of NV Pro is modulated by other viral proteins in the precursor forms and strongly influenced by cellular factors. NoroGLuc was also cleaved by Pro and Pro precursors generated from replication of NV stool RNA in transfected cells, resulting in a measurable increase of secreted GLuc. Truncation analysis revealed that the N-terminal membrane association domain of NV p41 is critical for NoroGLuc activity. Although designed for NV, a genogroup GI.1 norovirus, NoroGLuc also efficiently detects Pro activities from GII.3 and GII.4 noroviruses. At noncytotoxic concentrations, protease inhibitors ZnCl2 and Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) exhibited dose-dependent inhibitory effects on a GII.4 Pro by NoroGLuc assay. These results establish NoroGLuc as a pan-genogroup HuNoV protease reporter system that can be used for the study of HuNoV proteases and precursors, monitoring of viral RNA replication, and evaluation of antiviral agents.
Importance: Human noroviruses are the leading cause of epidemic gastroenteritis worldwide. Currently, there are no vaccines or antiviral drugs available to counter these highly contagious viruses. These viruses are currently noncultivatable in cell culture. Here, we report the development of a novel cell-based reporter system called NoroGLuc that can be used for studying norovirus replication and also for screening/evaluation of antiviral agents. This system is based on the fusion between viral protein p41 and a naturally secreted Gaussia luciferase (GLuc) with a cleavage site that can be recognized by the viral protease. Cleavage of this fusion protein by the viral protease results in the release and secretion of an active GLuc. Using NoroGLuc, we demonstrated a cell type-specific activity profile of the viral protease and its precursors and dose-dependent inhibitory effects of two protease inhibitors. This novel reporter system should be useful in probing norovirus replication and evaluating antiviral agents.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Replication of Human Norovirus RNA in Mammalian Cells Reveals Lack of Interferon Response.J Virol. 2016 Sep 12;90(19):8906-23. doi: 10.1128/JVI.01425-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466422 Free PMC article.
-
Characterization and inhibition of norovirus proteases of genogroups I and II using a fluorescence resonance energy transfer assay.Virology. 2012 Feb 20;423(2):125-33. doi: 10.1016/j.virol.2011.12.002. Epub 2011 Dec 24. Virology. 2012. PMID: 22200497 Free PMC article.
-
Conformational flexibility is a critical factor in designing broad-spectrum human norovirus protease inhibitors.J Virol. 2025 Feb 25;99(2):e0175724. doi: 10.1128/jvi.01757-24. Epub 2025 Jan 28. J Virol. 2025. PMID: 39873493 Free PMC article.
-
Norovirus Protease Structure and Antivirals Development.Viruses. 2021 Oct 14;13(10):2069. doi: 10.3390/v13102069. Viruses. 2021. PMID: 34696498 Free PMC article. Review.
-
Antiviral Drug Discovery: Norovirus Proteases and Development of Inhibitors.Viruses. 2019 Feb 25;11(2):197. doi: 10.3390/v11020197. Viruses. 2019. PMID: 30823509 Free PMC article. Review.
Cited by
-
Replication of Human Norovirus RNA in Mammalian Cells Reveals Lack of Interferon Response.J Virol. 2016 Sep 12;90(19):8906-23. doi: 10.1128/JVI.01425-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466422 Free PMC article.
-
Antiviral targets of human noroviruses.Curr Opin Virol. 2016 Jun;18:117-25. doi: 10.1016/j.coviro.2016.06.002. Epub 2016 Jun 17. Curr Opin Virol. 2016. PMID: 27318434 Free PMC article. Review.
-
Current tools for norovirus drug discovery.Expert Opin Drug Discov. 2016 Jun;11(6):529-41. doi: 10.1080/17460441.2016.1178231. Epub 2016 May 2. Expert Opin Drug Discov. 2016. PMID: 27108716 Free PMC article. Review.
-
Analysis of the overall development trends and hotspots in the research field of the human gut virome.Virol J. 2025 Jul 5;22(1):224. doi: 10.1186/s12985-025-02856-x. Virol J. 2025. PMID: 40618116 Free PMC article. Review.
-
Stepwise processing analyses of the single-turnover PCSK9 protease reveal its substrate sequence specificity and link clinical genotype to lipid phenotype.J Biol Chem. 2018 Feb 9;293(6):1875-1886. doi: 10.1074/jbc.RA117.000754. Epub 2017 Dec 19. J Biol Chem. 2018. PMID: 29259136 Free PMC article.
References
-
- Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. 2013. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 368:1121–1130. 10.1056/NEJMsa1206589 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

