Type III interferon attenuates a vesicular stomatitis virus-based vaccine vector
- PMID: 25008938
- PMCID: PMC4178858
- DOI: 10.1128/JVI.01910-14
Type III interferon attenuates a vesicular stomatitis virus-based vaccine vector
Abstract
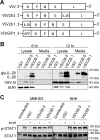
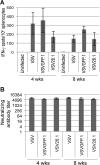
Vesicular stomatitis virus (VSV) has been extensively studied as a vaccine vector and oncolytic agent. Nevertheless, safety concerns have limited its widespread use in humans. The type III lambda interferon (IFN-λ) family of cytokines shares common signaling pathways with the IFN-α/β family and thus evokes similar antiviral activities. However, IFN-λ signals through a distinct receptor complex that is expressed in a cell type-specific manner, which restricts its activity to epithelial barriers, particularly those corresponding to the respiratory and gastrointestinal tracts. In this study, we determined how IFN-λ expression from recombinant VSV would influence vector replication, spread, and immunogenicity. We demonstrate that IFN-λ expression severely attenuates VSV in cell culture. In vivo, IFN-λ limits VSV replication in the mouse lung after intranasal administration and reduces virus spread to other organs. Despite this attenuation, however, the vector retains its capacity to induce protective CD8 T cell and antibody responses after a single immunization. These findings demonstrate a novel method of viral vector attenuation that could be used in both vaccine and oncolytic virus applications.
Importance: Viruses such as VSV that are used as vaccine vectors can induce protective T cell and antibody responses after a single dose. Additionally, IFN-λ is a potent antiviral agent that has certain advantages for clinical use compared to IFN-α/β, such as fewer patient side effects. Here, we demonstrate that IFN-λ attenuates VSV replication and spread following intranasal virus delivery but does not reduce the ability of VSV to induce potent protective immune responses. These findings demonstrate that the type III IFN family may have widespread applicability for improving the safety and efficacy of viral vaccine and oncolytic vectors.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Tesh RB, Peralta PH, Johnson KM. 1969. Ecologic studies of vesicular stomatitis virus. I. Prevalence of infection among animals and humans living in an area of endemic VSV activity. Am. J. Epidemiol. 90:255–261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

