Microtubule initiation from the nuclear surface controls cortical microtubule growth polarity and orientation in Arabidopsis thaliana
- PMID: 25008974
- PMCID: PMC4160572
- DOI: 10.1093/pcp/pcu094
Microtubule initiation from the nuclear surface controls cortical microtubule growth polarity and orientation in Arabidopsis thaliana
Abstract
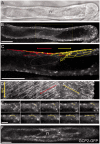
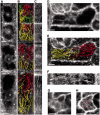
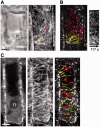
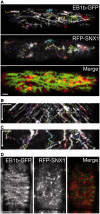
The nuclear envelope in plant cells has long been known to be a microtubule organizing center (MTOC), but its influence on microtubule organization in the cell cortex has been unclear. Here we show that nuclear MTOC activity favors the formation of longitudinal cortical microtubule (CMT) arrays. We used green fluorescent protein (GFP)-tagged gamma tubulin-complex protein 2 (GCP2) to identify nuclear MTOC activity and GFP-tagged End-Binding Protein 1b (EB1b) to track microtubule growth directions. We found that microtubules initiate from nuclei and enter the cortex in two directions along the long axis of the cell, creating bipolar longitudinal CMT arrays. Such arrays were observed in all cell types showing nuclear MTOC activity, including root hairs, recently divided cells in root tips, and the leaf epidermis. In order to confirm the causal nature of nuclei in bipolar array formation, we displaced nuclei by centrifugation, which generated a corresponding shift in the bipolarity split point. We also found that bipolar CMT arrays were associated with bidirectional trafficking of vesicular components to cell ends. Together, these findings reveal a conserved function of plant nuclear MTOCs and centrosomes/spindle pole bodies in animals and fungi, wherein all structures serve to establish polarities in microtubule growth.
Keywords: Arabidopsis; Cytoskeleton; Microtubule; Polarity; Root; Root hair.
© The Author 2014. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, et al. CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev. Cell. 2013;24:649–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

