A defined zebrafish line for high-throughput genetics and genomics: NHGRI-1
- PMID: 25009150
- PMCID: PMC4174928
- DOI: 10.1534/genetics.114.166769
A defined zebrafish line for high-throughput genetics and genomics: NHGRI-1
Abstract
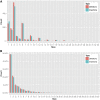
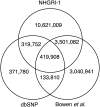
Substantial intrastrain variation at the nucleotide level complicates molecular and genetic studies in zebrafish, such as the use of CRISPRs or morpholinos to inactivate genes. In the absence of robust inbred zebrafish lines, we generated NHGRI-1, a healthy and fecund strain derived from founder parents we sequenced to a depth of ∼50×. Within this strain, we have identified the majority of the genome that matches the reference sequence and documented most of the variants. This strain has utility for many reasons, but in particular it will be useful for any researcher who needs to know the exact sequence (with all variants) of a particular genomic region or who wants to be able to robustly map sequences back to a genome with all possible variants defined.
Keywords: CRISPR; SNV; genome sequence; variants; zebrafish.
Copyright © 2014 by the Genetics Society of America.
Figures
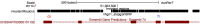
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

