Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells
- PMID: 25009205
- PMCID: PMC4120898
- DOI: 10.4049/jimmunol.1401243
Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells
Abstract
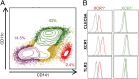
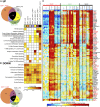
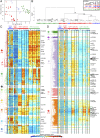
Human monocyte-derived dendritic cell (MoDC) have been used in the clinic with moderately encouraging results. Mouse XCR1(+) DC excel at cross-presentation, can be targeted in vivo to induce protective immunity, and share characteristics with XCR1(+) human DC. Assessment of the immunoactivation potential of XCR1(+) human DC is hindered by their paucity in vivo and by their lack of a well-defined in vitro counterpart. We report in this study a protocol generating both XCR1(+) and XCR1(-) human DC in CD34(+) progenitor cultures (CD34-DC). Gene expression profiling, phenotypic characterization, and functional studies demonstrated that XCR1(-) CD34-DC are similar to canonical MoDC, whereas XCR1(+) CD34-DC resemble XCR1(+) blood DC (bDC). XCR1(+) DC were strongly activated by polyinosinic-polycytidylic acid but not LPS, and conversely for MoDC. XCR1(+) DC and MoDC expressed strikingly different patterns of molecules involved in inflammation and in cross-talk with NK or T cells. XCR1(+) CD34-DC but not MoDC efficiently cross-presented a cell-associated Ag upon stimulation by polyinosinic-polycytidylic acid or R848, likewise to what was reported for XCR1(+) bDC. Hence, it is feasible to generate high numbers of bona fide XCR1(+) human DC in vitro as a model to decipher the functions of XCR1(+) bDC and as a potential source of XCR1(+) DC for clinical use.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures





References
-
- Adema G. J., de Vries I. J., Punt C. J., Figdor C. G. 2005. Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr. Opin. Immunol. 17: 170–174 - PubMed
-
- Plantinga M., Guilliams M., Vanheerswynghels M., Deswarte K., Branco-Madeira F., Toussaint W., Vanhoutte L., Neyt K., Killeen N., Malissen B., Hammad H., Lambrecht B. N. 2013. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38: 322‑335 - PubMed
-
- Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J., et al. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116: e74–e80 - PubMed
-
- Crozat K., Guiton R., Guilliams M., Henri S., Baranek T., Schwartz-Cornil I., Malissen B., Dalod M. 2010. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol. Rev. 234: 177–198 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

