Cortical GABAergic excitation contributes to epileptic activities around human glioma
- PMID: 25009229
- PMCID: PMC4409113
- DOI: 10.1126/scitranslmed.3008065
Cortical GABAergic excitation contributes to epileptic activities around human glioma
Abstract
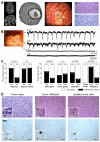
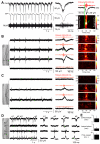
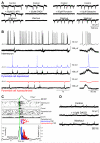
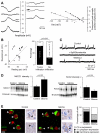
Brain gliomas are highly epileptogenic. Excitatory glutamatergic mechanisms are involved in the generation of epileptic activities in the neocortex surrounding gliomas. However, chloride homeostasis is known to be perturbed in glioma cells. Thus, the contribution of γ-aminobutyric acidergic (GABAergic) mechanisms that depend on intracellular chloride merits closer study. We studied the occurrence, networks, cells, and signaling basis of epileptic activities in neocortical slices from the peritumoral surgical margin resected around human brain gliomas. Postoperative glioma tissue from 69% of patients spontaneously generated interictal-like discharges, synchronized, with a high-frequency oscillation signature, in superficial layers of neocortex around areas of glioma infiltration. Interictal-like events depended both on glutamatergic AMPA receptor-mediated transmission and on depolarizing GABAergic signaling. GABA released by interneurons depolarized 65% of pyramidal cells, in which chloride homeostasis was perturbed because of changes in expression of neuronal chloride cotransporters: KCC2 (K-Cl cotransporter 2) was reduced by 42% and expression of NKCC1 (Na-K-2Cl cotransporter 1) increased by 144%. Ictal-like activities were initiated by convulsant stimuli exclusively in these epileptogenic areas. This study shows that epileptic activities are sustained by excitatory effects of GABA in human peritumoral neocortex, as reported in temporal lobe epilepsies, suggesting that both glutamate and GABA signaling and cellular chloride regulation processes, all also involved in oncogenesis as already shown, induce an imbalance between synaptic excitation and inhibition underlying epileptic discharges in glioma patients. Thus, the control of chloride in neurons and glioma cells may provide a therapeutic target for patients with epileptogenic gliomas.
Copyright © 2014, American Association for the Advancement of Science.
Figures
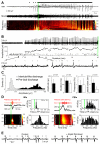
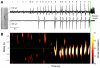
References
-
- van Breemen MSM, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet neurology. 2007;6:421–430. - PubMed
-
- Pallud J, Capelle L, Huberfeld G. Tumoral epilepsy. How does it happen? Epilepsia. 2013 - PubMed
-
- Hirsch JF, Buisson-Ferey J, Sachs M, Hirsch JC, Scherrer J. [Electrocorticogram and unitary activites with expanding lesions in man] Electroencephalogr Clin Neurophysiol. 1966;21:417–428. - PubMed
-
- Berger MS, Ghatan S, Haglund MM, Dobbins J, Ojemann GA. Low-grade gliomas associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. Journal of Neurosurgery. 1993;79:62–69. - PubMed
-
- Haglund MM, Berger MS, Kunkel DD, Franck JE, Ghatan S, Ojemann GA. Changes in gamma-aminobutyric acid and somatostatin in epileptic cortex associated with low-grade gliomas. Journal of Neurosurgery. 1992;77:209–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

