Calsyntenin-1 regulates axon branching and endosomal trafficking during sensory neuron development in vivo
- PMID: 25009257
- PMCID: PMC4087204
- DOI: 10.1523/JNEUROSCI.0561-14.2014
Calsyntenin-1 regulates axon branching and endosomal trafficking during sensory neuron development in vivo
Abstract
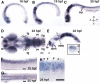
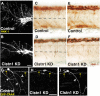
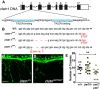
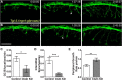
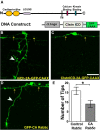
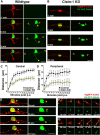
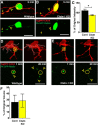
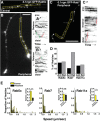
Precise regulation of axon branching is crucial for neuronal circuit formation, yet the mechanisms that control branch formation are not well understood. Moreover, the highly complex morphology of neurons makes them critically dependent on protein/membrane trafficking and transport systems, although the functions for membrane trafficking in neuronal morphogenesis are largely undefined. Here we identify a kinesin adaptor, Calsyntenin-1 (Clstn-1), as an essential regulator of axon branching and neuronal compartmentalization in vivo. We use morpholino knockdown and a Clstn-1 mutant to show that Clstn-1 is required for formation of peripheral but not central sensory axons, and for peripheral axon branching in zebrafish. We used live imaging of endosomal trafficking in vivo to show that Clstn-1 regulates transport of Rab5-containing endosomes from the cell body to specific locations of developing axons. Our results suggest a model in which Clstn-1 patterns separate axonal compartments and define their ability to branch by directing trafficking of specific endosomes.
Keywords: axon branching; calsyntenin; endosome; polarity; trafficking; zebrafish.
Copyright © 2014 the authors 0270-6474/14/349235-14$15.00/0.
Figures

Similar articles
-
The Kinesin Adaptor Calsyntenin-1 Organizes Microtubule Polarity and Regulates Dynamics during Sensory Axon Arbor Development.Front Cell Neurosci. 2017 Apr 20;11:107. doi: 10.3389/fncel.2017.00107. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28473757 Free PMC article.
-
In vivo imaging of cell behaviors and F-actin reveals LIM-HD transcription factor regulation of peripheral versus central sensory axon development.Neural Dev. 2011 May 27;6:27. doi: 10.1186/1749-8104-6-27. Neural Dev. 2011. PMID: 21619654 Free PMC article.
-
KLC4 shapes axon arbors during development and mediates adult behavior.Elife. 2022 Oct 12;11:e74270. doi: 10.7554/eLife.74270. Elife. 2022. PMID: 36222498 Free PMC article.
-
Harnessing the power of the endosome to regulate neural development.Neuron. 2012 May 10;74(3):440-51. doi: 10.1016/j.neuron.2012.04.015. Neuron. 2012. PMID: 22578496 Free PMC article. Review.
-
Axon guidance receptors: Endocytosis, trafficking and downstream signaling from endosomes.Prog Neurobiol. 2021 Mar;198:101916. doi: 10.1016/j.pneurobio.2020.101916. Epub 2020 Sep 28. Prog Neurobiol. 2021. PMID: 32991957 Review.
Cited by
-
Nerve growth factor promotes reorganization of the axonal microtubule array at sites of axon collateral branching.Dev Neurobiol. 2015 Dec;75(12):1441-61. doi: 10.1002/dneu.22294. Epub 2015 May 27. Dev Neurobiol. 2015. PMID: 25846486 Free PMC article.
-
Calsyntenin-1 Promotes Doxorubicin-induced Dilated Cardiomyopathy in Rats.Cardiovasc Drugs Ther. 2024 Apr;38(2):237-252. doi: 10.1007/s10557-022-07389-x. Epub 2022 Nov 9. Cardiovasc Drugs Ther. 2024. PMID: 36350487 Free PMC article.
-
Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats.Neuroscience. 2015 Feb 12;286:87-96. doi: 10.1016/j.neuroscience.2014.11.030. Epub 2014 Nov 26. Neuroscience. 2015. PMID: 25463516 Free PMC article.
-
Cytoskeleton and Membrane Organization at Axon Branches.Front Cell Dev Biol. 2021 Sep 1;9:707486. doi: 10.3389/fcell.2021.707486. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34540830 Free PMC article. Review.
-
Extracellular Vesicle Protein Expression in Doped Bioactive Glasses: Further Insights Applying Anomaly Detection.Int J Mol Sci. 2024 Mar 21;25(6):3560. doi: 10.3390/ijms25063560. Int J Mol Sci. 2024. PMID: 38542533 Free PMC article.
References
-
- Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T. Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid beta-protein precursor metabolism. J Biol Chem. 2003;278:49448–49458. doi: 10.1074/jbc.M306024200. - DOI - PubMed
-
- Araki Y, Kawano T, Taru H, Saito Y, Wada S, Miyamoto K, Kobayashi H, Ishikawa HO, Ohsugi Y, Yamamoto T, Matsuno K, Kinjo M, Suzuki T. The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. EMBO J. 2007;26:1475–1486. doi: 10.1038/sj.emboj.7601609. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
