The role of the hypothalamic paraventricular nucleus and the organum vasculosum lateral terminalis in the control of sodium appetite in male rats
- PMID: 25009258
- PMCID: PMC4087205
- DOI: 10.1523/JNEUROSCI.3979-13.2014
The role of the hypothalamic paraventricular nucleus and the organum vasculosum lateral terminalis in the control of sodium appetite in male rats
Abstract
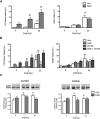
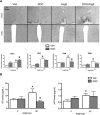
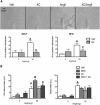
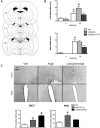
Angiotensin II (AngII) and aldosterone cooperate centrally to produce a robust sodium appetite. The intracellular signaling and circuitry that underlie this interaction remain unspecified. Male rats pretreated with both deoxycorticosterone (DOC; a synthetic precursor of aldosterone) and central AngII exhibited a marked sodium intake, as classically described. Disruption of inositol trisphosphate signaling, but not extracellular-regulated receptor kinase 1 and 2 signaling, prevented the cooperativity of DOC and AngII on sodium intake. The pattern of expression of the immediate early gene product cFos was used to identify key brain regions that may underlie this behavior. In the paraventricular nuclei (PVN) of the hypothalamus, DOC pretreatment diminished both AngII-induced cFos induction and neurosecretion of oxytocin, a peptide expressed in the PVN. Conversely, in the organum vasculosum lateral terminalis (OVLT), DOC pretreatment augmented cFos expression. Immunohistochemistry identified a substantial presence of oxytocin fibers in the OVLT. In addition, when action potentials in the PVN were inhibited with intraparenchymal lidocaine, AngII-induced sodium ingestion was exaggerated. Intriguingly, this treatment also increased the number of neurons in the OVLT expressing AngII-induced cFos. Collectively, these results suggest that the behavioral cooperativity between DOC and AngII involves the alleviation of an inhibitory oxytocin signal, possibly relayed directly from the PVN to the OVLT.
Keywords: aldosterone; angiotensin; oxytocin; receptor signaling; sodium appetite.
Copyright © 2014 the authors 0270-6474/14/349249-12$15.00/0.
Figures

References
-
- Beresford MJ, Fitzsimons JT. Intracerebroventricular angiotensin II-induced thirst and sodium appetite in rat are blocked by the AT1 receptor antagonist, losartan (Dup753), but not by the AT2 antagonist, CGP 42112A. Exp Physiol. 1992;77:761–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
