Huntingtin is required for normal excitatory synapse development in cortical and striatal circuits
- PMID: 25009276
- PMCID: PMC4087216
- DOI: 10.1523/JNEUROSCI.4699-13.2014
Huntingtin is required for normal excitatory synapse development in cortical and striatal circuits
Abstract
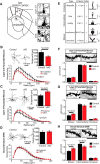
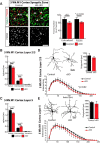
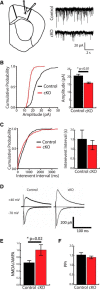
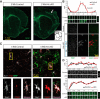
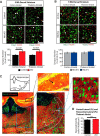
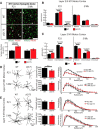
Huntington's disease (HD) is a neurodegenerative disease caused by the expansion of a poly-glutamine (poly-Q) stretch in the huntingtin (Htt) protein. Gain-of-function effects of mutant Htt have been extensively investigated as the major driver of neurodegeneration in HD. However, loss-of-function effects of poly-Q mutations recently emerged as potential drivers of disease pathophysiology. Early synaptic problems in the excitatory cortical and striatal connections have been reported in HD, but the role of Htt protein in synaptic connectivity was unknown. Therefore, we investigated the role of Htt in synaptic connectivity in vivo by conditionally silencing Htt in the developing mouse cortex. When cortical Htt function was silenced, cortical and striatal excitatory synapses formed and matured at an accelerated pace through postnatal day 21 (P21). This exuberant synaptic connectivity was lost over time in the cortex, resulting in the deterioration of synapses by 5 weeks. Synaptic decline in the cortex was accompanied with layer- and region-specific reactive gliosis without cell loss. To determine whether the disease-causing poly-Q mutation in Htt affects synapse development, we next investigated the synaptic connectivity in a full-length knock-in mouse model of HD, the zQ175 mouse. Similar to the cortical conditional knock-outs, we found excessive excitatory synapse formation and maturation in the cortices of P21 zQ175, which was lost by 5 weeks. Together, our findings reveal that cortical Htt is required for the correct establishment of cortical and striatal excitatory circuits, and this function of Htt is lost when the mutant Htt is present.
Keywords: corticostriatal connections; excitatory synapses; huntingtin; reactive gliosis; synapse maturation; synaptogenesis.
Copyright © 2014 the authors 0270-6474/14/349455-18$15.00/0.
Figures




Similar articles
-
Impaired development of cortico-striatal synaptic connectivity in a cell culture model of Huntington's disease.Neurobiol Dis. 2016 Mar;87:80-90. doi: 10.1016/j.nbd.2015.12.009. Epub 2015 Dec 19. Neurobiol Dis. 2016. PMID: 26711622
-
Differential changes in thalamic and cortical excitatory synapses onto striatal spiny projection neurons in a Huntington disease mouse model.Neurobiol Dis. 2016 Feb;86:62-74. doi: 10.1016/j.nbd.2015.11.020. Epub 2015 Nov 24. Neurobiol Dis. 2016. PMID: 26621114
-
Altering cortical input unmasks synaptic phenotypes in the YAC128 cortico-striatal co-culture model of Huntington disease.BMC Biol. 2018 Jun 27;16(1):58. doi: 10.1186/s12915-018-0526-3. BMC Biol. 2018. PMID: 29945611 Free PMC article.
-
[Huntington's disease: cellular and molecular basis of pathology].Zh Vyssh Nerv Deiat Im I P Pavlova. 2014 Jul-Aug;64(4):359-75. Zh Vyssh Nerv Deiat Im I P Pavlova. 2014. PMID: 25723022 Review. Russian.
-
Selective degeneration in YAC mouse models of Huntington disease.Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review.
Cited by
-
Neurochemical correlates of synapse density in a Huntington's disease mouse model.J Neurochem. 2023 Jan;164(2):226-241. doi: 10.1111/jnc.15714. Epub 2022 Nov 11. J Neurochem. 2023. PMID: 36272099 Free PMC article.
-
The effects of huntingtin-lowering: what do we know so far?Degener Neurol Neuromuscul Dis. 2019 Mar 8;9:3-17. doi: 10.2147/DNND.S163808. eCollection 2019. Degener Neurol Neuromuscul Dis. 2019. PMID: 30881191 Free PMC article. Review.
-
CD40L Reverse Signaling Influences Dendrite Spine Morphology and Expression of PSD-95 and Rho Small GTPases.Front Cell Dev Biol. 2020 Apr 28;8:254. doi: 10.3389/fcell.2020.00254. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32411702 Free PMC article.
-
Striatal Projection Neurons Require Huntingtin for Synaptic Connectivity and Survival.Cell Rep. 2020 Jan 21;30(3):642-657.e6. doi: 10.1016/j.celrep.2019.12.069. Cell Rep. 2020. PMID: 31968243 Free PMC article.
-
Allele-Specific Knockdown of Mutant Huntingtin Protein via Editing at Coding Region Single Nucleotide Polymorphism Heterozygosities.Hum Gene Ther. 2022 Jan;33(1-2):25-36. doi: 10.1089/hum.2020.323. Hum Gene Ther. 2022. PMID: 34376056 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
