CASK and CaMKII function in Drosophila memory
- PMID: 25009461
- PMCID: PMC4070058
- DOI: 10.3389/fnins.2014.00178
CASK and CaMKII function in Drosophila memory
Abstract
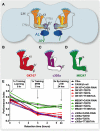
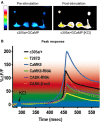
Calcium (Ca(2+)) and Calmodulin (CaM)-dependent serine/threonine kinase II (CaMKII) plays a central role in synaptic plasticity and memory due to its ability to phosphorylate itself and regulate its own kinase activity. Autophosphorylation at threonine 287 (T287) switches CaMKII to a Ca(2+) independent and constitutively active state replicated by overexpression of a phosphomimetic CaMKII-T287D transgene or blocked by expression of a T287A transgene. A second pair of sites, T306 T307 in the CaM binding region once autophosphorylated, prevents CaM binding and inactivates the kinase during synaptic plasticity and memory, and can be blocked by a TT306/7AA transgene. Recently the synaptic scaffolding molecule called CASK (Ca(2+)/CaM-associated serine kinase) has been shown to control both sets of CaMKII autophosphorylation events during neuronal growth, Ca(2+) signaling and memory in Drosophila. Deletion of either full length CASK or just its CaMK-like and L27 domains removed middle-term memory (MTM) and long-term memory (LTM), with CASK function in the α'/ß' mushroom body neurons being required for memory. In a similar manner directly changing the levels of CaMKII autophosphorylation (T287D, T287A, or TT306/7AA) in the α'/ß' neurons also removed MTM and LTM. In the CASK null mutant expression of either the Drosophila or human CASK transgene in the α'/ß' neurons was found to completely rescue memory, confirming that CASK signaling in α'/β' neurons is necessary and sufficient for Drosophila memory formation and that the neuronal function of CASK is conserved between Drosophila and human. Expression of human CASK in Drosophila also rescued the effect of CASK deletion on the activity state of CaMKII, suggesting that human CASK may also regulate CaMKII autophosphorylation. Mutations in human CASK have recently been shown to result in intellectual disability and neurological defects suggesting a role in plasticity and learning possibly via regulation of CaMKII autophosphorylation.
Keywords: CASK; CaMKII; Drosophila; autophosphorylation; calcium imaging; disease model; memory; mushroom body.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

