Revisiting enigmatic cortical calretinin-expressing interneurons
- PMID: 25009470
- PMCID: PMC4067953
- DOI: 10.3389/fnana.2014.00052
Revisiting enigmatic cortical calretinin-expressing interneurons
Abstract
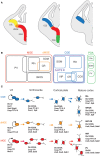
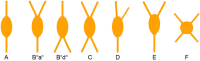
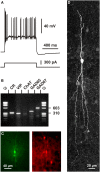
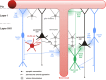
Cortical calretinin (CR)-expressing interneurons represent a heterogeneous subpopulation of about 10-30% of GABAergic interneurons, which altogether total ca. 12-20% of all cortical neurons. In the rodent neocortex, CR cells display different somatodendritic morphologies ranging from bipolar to multipolar but the bipolar cells and their variations dominate. They are also diverse at the molecular level as they were shown to express numerous neuropeptides in different combinations including vasoactive intestinal polypeptide (VIP), cholecystokinin (CCK), neurokinin B (NKB) corticotrophin releasing factor (CRF), enkephalin (Enk) but also neuropeptide Y (NPY) and somatostatin (SOM) to a lesser extent. CR-expressing interneurons exhibit different firing behaviors such as adapting, bursting or irregular. They mainly originate from the caudal ganglionic eminence (CGE) but a subpopulation also derives from the dorsal part of the medial ganglionic eminence (MGE). Cortical GABAergic CR-expressing interneurons can be divided in two main populations: VIP-bipolar interneurons deriving from the CGE and SOM-Martinotti-like interneurons originating in the dorsal MGE. Although bipolar cells account for the majority of CR-expressing interneurons, the roles they play in cortical neuronal circuits and in the more general metabolic physiology of the brain remained elusive and enigmatic. The aim of this review is, firstly, to provide a comprehensive view of the morphological, molecular and electrophysiological features defining this cell type. We will, secondly, also summarize what is known about their place in the cortical circuit, their modulation by subcortical afferents and the functional roles they might play in neuronal processing and energy metabolism.
Keywords: embryonic and fetal development; neocortex; neocortical circuits; neuroenergetics; neuropeptides.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

