The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities
- PMID: 25009507
- PMCID: PMC4070480
- DOI: 10.3389/fphys.2014.00238
The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities
Abstract
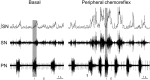
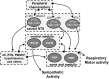
It is well known that breathing introduces rhythmical oscillations in the heart rate and arterial pressure levels. Sympathetic oscillations coupled to the respiratory activity have been suggested as an important homeostatic mechanism optimizing tissue perfusion and blood gas uptake/delivery. This respiratory-sympathetic coupling is strengthened in conditions of blood gas challenges (hypoxia and hypercapnia) as a result of the synchronized activation of brainstem respiratory and sympathetic neurons, culminating with the emergence of entrained cardiovascular and respiratory reflex responses. Studies have proposed that the ventrolateral region of the medulla oblongata is a major site of synaptic interaction between respiratory and sympathetic neurons. However, other brainstem regions also play a relevant role in the patterning of respiratory and sympathetic motor outputs. Recent findings suggest that the neurons of the nucleus of the solitary tract (NTS), in the dorsal medulla, are essential for the processing and coordination of respiratory and sympathetic responses to hypoxia. The NTS is the first synaptic station of the cardiorespiratory afferent inputs, including peripheral chemoreceptors, baroreceptors and pulmonary stretch receptors. The synaptic profile of the NTS neurons receiving the excitatory drive from afferent inputs is complex and involves distinct neurotransmitters, including glutamate, ATP and acetylcholine. In the present review we discuss the role of the NTS circuitry in coordinating sympathetic and respiratory reflex responses. We also analyze the neuroplasticity of NTS neurons and their contribution for the development of cardiorespiratory dysfunctions, as observed in neurogenic hypertension, obstructive sleep apnea and metabolic disorders.
Keywords: NTS; cardiorespiratory coupling; chemoreflex; neurotransmission; respiration; sympathetic activity.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

