N-(p-Coumaryol)-Tryptamine Suppresses the Activation of JNK/c-Jun Signaling Pathway in LPS-Challenged RAW264.7 Cells
- PMID: 25009700
- PMCID: PMC4060082
- DOI: 10.4062/biomolther.2014.013
N-(p-Coumaryol)-Tryptamine Suppresses the Activation of JNK/c-Jun Signaling Pathway in LPS-Challenged RAW264.7 Cells
Abstract
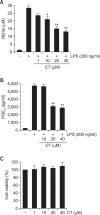
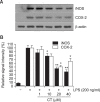
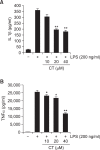
N-(p-Coumaryol) tryptamine (CT), a phenolic amide, has been reported to exhibit anti-oxidant and anti-inflammatory activities. However, the underlying mechanism by which CT exerts its pharmacological properties has not been clearly demonstrated. The objective of this study is to elucidate the anti-inflammatory mechanism of CT in lipopolysaccharide (LPS)-challenged RAW264.7 macrophage cells. CT significantly inhibited LPS-induced extracellular secretion of pro-inflammatory mediators such as nitric oxide (NO) and PGE2, and protein expressions of iNOS and COX-2. In addition, CT significantly suppressed LPS-induced secretion of pro-inflammatory cytokines such as TNF-α and IL-1β. To elucidate the underlying anti-inflammatory mechanism of CT, involvement of MAPK and Akt signaling pathways was examined. CT significantly attenuated LPS-induced activation of JNK/c-Jun, but not ERK and p38, in a concentration-dependent manner. Interestingly, CT appeared to suppress LPS-induced Akt phosphorylation. However, JNK inhibition, but not Akt inhibition, resulted in the suppression of LPS-induced responses, suggesting that JNK/c-Jun signaling pathway significantly contributes to LPS-induced inflammatory responses and that LPS-induced Akt phosphorylation might be a compensatory response to a stress condition. Taken together, the present study clearly demonstrates CT exerts anti-inflammatory activity through the suppression of JNK/c-Jun signaling pathway in LPS-challenged RAW264.7 macrophage cells.
Keywords: COX-2; JNK; Lipopolysaccharide; N-(p-Coumaroyl) tryptamine; RAW 264.7 cells; c-Jun; iNOS.
Figures
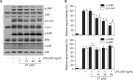
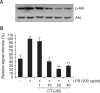

Similar articles
-
Quercetin-3-O-β-D-Glucuronide Suppresses Lipopolysaccharide-Induced JNK and ERK Phosphorylation in LPS-Challenged RAW264.7 Cells.Biomol Ther (Seoul). 2016 Nov 1;24(6):610-615. doi: 10.4062/biomolther.2016.026. Biomol Ther (Seoul). 2016. PMID: 27257013 Free PMC article.
-
Phosphorylation of Akt Mediates Anti-Inflammatory Activity of 1-p-Coumaroyl β-D-Glucoside Against Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells.Korean J Physiol Pharmacol. 2014 Feb;18(1):79-86. doi: 10.4196/kjpp.2014.18.1.79. Epub 2014 Feb 13. Korean J Physiol Pharmacol. 2014. PMID: 24634601 Free PMC article.
-
Isorhamnetin-3-O-Glucuronide Suppresses JNK and p38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells.Drug Dev Res. 2016 May;77(3):143-51. doi: 10.1002/ddr.21301. Drug Dev Res. 2016. PMID: 27113811
-
Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-κB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells.Biomol Ther (Seoul). 2013 May 30;21(3):216-21. doi: 10.4062/biomolther.2013.023. Biomol Ther (Seoul). 2013. PMID: 24265867 Free PMC article.
-
Apocynin Suppresses Lipopolysaccharide-Induced Inflammatory Responses Through the Inhibition of MAP Kinase Signaling Pathway in RAW264.7 Cells.Drug Dev Res. 2016 Sep;77(6):271-7. doi: 10.1002/ddr.21321. Epub 2016 Aug 4. Drug Dev Res. 2016. PMID: 27488478
Cited by
-
Potential roles of the interactions between gut microbiota and metabolites in LPS-induced intrauterine inflammation (IUI) and associated preterm birth (PTB).J Transl Med. 2024 Jan 2;22(1):7. doi: 10.1186/s12967-023-04603-8. J Transl Med. 2024. PMID: 38167140 Free PMC article.
-
Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review.Molecules. 2017 Feb 13;22(2):281. doi: 10.3390/molecules22020281. Molecules. 2017. PMID: 28208818 Free PMC article. Review.
-
Quercetin-3-O-β-D-Glucuronide Suppresses Lipopolysaccharide-Induced JNK and ERK Phosphorylation in LPS-Challenged RAW264.7 Cells.Biomol Ther (Seoul). 2016 Nov 1;24(6):610-615. doi: 10.4062/biomolther.2016.026. Biomol Ther (Seoul). 2016. PMID: 27257013 Free PMC article.
-
Anti-Inflammatory Effect of Turbo cornutus Viscera Ethanolic Extract against Lipopolysaccharide-Stimulated Inflammatory Response via the Regulation of the JNK/NF-kB Signaling Pathway in Murine Macrophage RAW 264.7 Cells and a Zebrafish Model: A Preliminary Study.Foods. 2022 Jan 27;11(3):364. doi: 10.3390/foods11030364. Foods. 2022. PMID: 35159514 Free PMC article.
References
-
- Andrianaivoravelona JO, Terreaux C, Sahpaz S, Rasolondramanitra J, Hostettmann K. A phenolic glycoside and N-(p-coumaroyl)-tryptamine from Ravensara anisata. Phytochemistry. 1999;52:1145–1148. - PubMed
-
- Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35:24–27. - PubMed
-
- Dilshara MG, Jayasooriya RG, Lee S, Jeong JB, Seo YT, Choi YH, Jeong JW, Jang YP, Jeong YK, Kim GY. Water extract of processed Hydrangea macrophylla (Thunb.) Ser. leaf attenuates the expression of pro-inflammatory mediators by suppressing Akt-mediated NF-kappaB activation. Environ Toxicol Pharmacol. 2013;35:311–319. - PubMed
-
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. - PubMed
-
- Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Park MK, Chang KC. beta(1)-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol. 2011;82:769–777. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

