HMGB1 in health and disease
- PMID: 25010388
- PMCID: PMC4254084
- DOI: 10.1016/j.mam.2014.05.001
HMGB1 in health and disease
Abstract
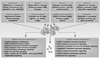
Complex genetic and physiological variations as well as environmental factors that drive emergence of chromosomal instability, development of unscheduled cell death, skewed differentiation, and altered metabolism are central to the pathogenesis of human diseases and disorders. Understanding the molecular bases for these processes is important for the development of new diagnostic biomarkers, and for identifying new therapeutic targets. In 1973, a group of non-histone nuclear proteins with high electrophoretic mobility was discovered and termed high-mobility group (HMG) proteins. The HMG proteins include three superfamilies termed HMGB, HMGN, and HMGA. High-mobility group box 1 (HMGB1), the most abundant and well-studied HMG protein, senses and coordinates the cellular stress response and plays a critical role not only inside of the cell as a DNA chaperone, chromosome guardian, autophagy sustainer, and protector from apoptotic cell death, but also outside the cell as the prototypic damage associated molecular pattern molecule (DAMP). This DAMP, in conjunction with other factors, thus has cytokine, chemokine, and growth factor activity, orchestrating the inflammatory and immune response. All of these characteristics make HMGB1 a critical molecular target in multiple human diseases including infectious diseases, ischemia, immune disorders, neurodegenerative diseases, metabolic disorders, and cancer. Indeed, a number of emergent strategies have been used to inhibit HMGB1 expression, release, and activity in vitro and in vivo. These include antibodies, peptide inhibitors, RNAi, anti-coagulants, endogenous hormones, various chemical compounds, HMGB1-receptor and signaling pathway inhibition, artificial DNAs, physical strategies including vagus nerve stimulation and other surgical approaches. Future work further investigating the details of HMGB1 localization, structure, post-translational modification, and identification of additional partners will undoubtedly uncover additional secrets regarding HMGB1's multiple functions.
Keywords: Biology; DAMP; Disease; HMGB1.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures







References
-
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273–1275. - PubMed
-
- Abarbanell AM, Hartley JA, Herrmann JL, Weil BR, Wang Y, Manukyan MC, Poynter JA, Meldrum DR. Exogenous high-mobility group box 1 improves myocardial recovery after acute global ischemia/reperfusion injury. Surgery. 2011;149(3):329–335. - PubMed
-
- Abdul-Razzak KK, Denton ML, Cox DJ, Reeck GR. Isolation and characterization of folded fragments released by Staphylococcal aureus proteinase from the non-histone chromosomal protein HMG-1. Biochim Biophys Acta. 1989;996(1–2):125–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

