Development of fatal intestinal inflammation in MyD88 deficient mice co-infected with helminth and bacterial enteropathogens
- PMID: 25010669
- PMCID: PMC4091940
- DOI: 10.1371/journal.pntd.0002987
Development of fatal intestinal inflammation in MyD88 deficient mice co-infected with helminth and bacterial enteropathogens
Abstract
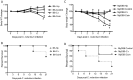
Infections with intestinal helminth and bacterial pathogens, such as enteropathogenic Escherichia coli, continue to be a major global health threat for children. To determine whether and how an intestinal helminth parasite, Heligomosomoides polygyrus, might impact the TLR signaling pathway during the response to a bacterial enteropathogen, MyD88 knockout and wild-type C57BL/6 mice were infected with H. polygyrus, the bacterial enteropathogen Citrobacter rodentium, or both. We found that MyD88 knockout mice co-infected with H. polygyrus and C. rodentium developed more severe intestinal inflammation and elevated mortality compared to the wild-type mice. The enhanced susceptibility to C. rodentium, intestinal injury and mortality of the co-infected MyD88 knockout mice were found to be associated with markedly reduced intestinal phagocyte recruitment, decreased expression of the chemoattractant KC, and a significant increase in bacterial translocation. Moreover, the increase in bacterial infection and disease severity were found to be correlated with a significant downregulation of antimicrobial peptide expression in the intestinal tissue in co-infected MyD88 knockout mice. Our results suggest that the MyD88 signaling pathway plays a critical role for host defense and survival during helminth and enteric bacterial co-infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice.Infect Immun. 2005 Sep;73(9):5468-81. doi: 10.1128/IAI.73.9.5468-5481.2005. Infect Immun. 2005. PMID: 16113263 Free PMC article.
-
Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses.Infect Immun. 2014 Sep;82(9):3753-63. doi: 10.1128/IAI.02045-14. Epub 2014 Jun 23. Infect Immun. 2014. PMID: 24958710 Free PMC article.
-
MyD88 signaling in dendritic cells and the intestinal epithelium controls immunity against intestinal infection with C. rodentium.PLoS Pathog. 2017 May 16;13(5):e1006357. doi: 10.1371/journal.ppat.1006357. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28520792 Free PMC article.
-
TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium.J Immunol. 2007 Jul 1;179(1):566-77. doi: 10.4049/jimmunol.179.1.566. J Immunol. 2007. PMID: 17579078
-
Helminth-primed dendritic cells alter the host response to enteric bacterial infection.J Immunol. 2006 Jan 1;176(1):472-83. doi: 10.4049/jimmunol.176.1.472. J Immunol. 2006. PMID: 16365440 Free PMC article.
Cited by
-
Macrophage origin limits functional plasticity in helminth-bacterial co-infection.PLoS Pathog. 2017 Mar 23;13(3):e1006233. doi: 10.1371/journal.ppat.1006233. eCollection 2017 Mar. PLoS Pathog. 2017. PMID: 28334040 Free PMC article.
-
The impact of helminth-induced immunity on infection with bacteria or viruses.Vet Res. 2023 Oct 3;54(1):87. doi: 10.1186/s13567-023-01216-3. Vet Res. 2023. PMID: 37789420 Free PMC article. Review.
-
Abdominal Distension and Escherichia coli Peritonitis in Mice Lacking Myeloid Differentiation Factor 88.Comp Med. 2015 Apr;65(2):123-6. Comp Med. 2015. PMID: 25926397 Free PMC article.
-
Toxoplasma Co-infection Prevents Th2 Differentiation and Leads to a Helminth-Specific Th1 Response.Front Cell Infect Microbiol. 2017 Jul 25;7:341. doi: 10.3389/fcimb.2017.00341. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28791259 Free PMC article.
References
-
- Hotez P, Mistry N, Rubinstein J, Sachs J (2011) Integrating neglected tropical diseases into AIDS, tuberculosis, and malaria control. The New England journal of medicine 364: 2086–2089. - PubMed
-
- Strober W (2009) The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity 31: 377–388. - PubMed
-
- Kaisho T, Akira S (2004) Pleiotropic function of Toll-like receptors. Microbes Infect 6: 388–1394. - PubMed
-
- Fitzgerald K, Palsson-McDermott E, Bowie A, Jefferies C, Mansell A, et al. (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413: 78–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

