Combination of tocolytic agents for inhibiting preterm labour
- PMID: 25010869
- PMCID: PMC10657484
- DOI: 10.1002/14651858.CD006169.pub2
Combination of tocolytic agents for inhibiting preterm labour
Abstract
Background: Preterm birth represents the single largest cause of mortality and morbidity for newborns and a major cause of morbidity for pregnant women. Tocolytic agents include a wide range of drugs that can inhibit labour to prolong pregnancy. This may gain time to allow the fetus to mature further before being born, permit antenatal corticosteroid administration for lung maturation, and allow time for intra-uterine transfer to a hospital with neonatal intensive care facilities. However, some tocolytic drugs are associated with severe side effects. Combinations of tocolytic drugs may be more effective over single tocolytic agents or no intervention, without adversely affecting the mother or neonate.
Objectives: To assess the effects on maternal, fetal and neonatal outcomes of any combination of tocolytic drugs for the treatment of preterm labour when compared with any other treatment, no treatment or placebo.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2014) and reference lists of retrieved studies.
Selection criteria: We included randomised controlled trials comparing a combination of tocolytic agents, administered by any route or any dose, for inhibiting preterm labour versus any other treatment (including other combinations of tocolytics or single tocolytics), no intervention or placebo.
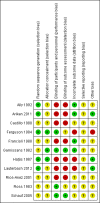
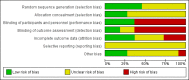
Data collection and analysis: Two review authors independently assessed study reports for eligibility, carried out data extraction and assessed risk of bias.
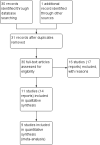
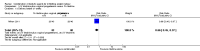
Main results: Eleven studies met our inclusion criteria. Two studies did not report any outcome data relevant to the review, so the results of the review are based on nine trials that contributed data. Primary outcomes were perinatal mortality, serious maternal or infant outcomes, adverse drug reactions, birth before 48 hours of trial entry, birth before 34 weeks' gestation and preterm neonates delivered without a full course of antenatal steroids completed 24 hours before birth. The quality of evidence in included trials was mixed; only three of the trials were placebo controlled.The included trials examined seven different comparisons: intravenous (IV) ritodrine plus oral or IV magnesium (sulphate or gluconate) versus IV ritodrine alone (three trials, 231 women); IV ritodrine plus indomethacin suppositories versus IV ritodrine alone (one trial, 208 women); IV ritodrine plus vaginal progesterone versus IV ritodrine alone (one trial, 83 women); IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone (one trial, 24 women); IV fenoterol plus oral naproxen versus IV fenoterol alone (one trial, 72 women); oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol (one trial, 125 women); and, IV terbutaline plus oral metoprolol versus IV terbutaline alone (one trial, 17 women). Few studies with small numbers of women were available for each comparison, hence very little data were pooled in meta-analysis. In all trials, not many of the primary outcomes were reported.Three trials examined intravenous (IV) ritodrine plus IV or oral magnesium (sulphate or gluconate) compared with IV ritodrine alone. One study, with 41 women, reported more adverse drug reactions in the group receiving the combined tocolytics (risk ratio (RR) 7.79, 95% confidence interval (CI) 1.11 to 54.80). Two trials reported discontinuation of therapy due to severe side effects (results were not combined due to high statistical heterogeneity, I² = 83%); one trial reported increased severe side effects in the group receiving IV ritodrine alone (RR 7.79, 95% CI 1.11 to 54.80, 41 women); in the other trial there was no clear difference between groups (RR 0.23, 95% CI 0.03 to 1.97, 107 women). Other primary outcomes were not reported.One trial assessed IV ritodrine plus indomethacin suppositories versus IV ritodrine alone. There were no significant differences between groups for perinatal mortality or serious neonatal morbidity. Results for other primary outcomes were not reported.There were no significant differences between groups receiving IV ritodrine plus vaginal progesterone compared with IV ritodrine alone for most outcomes reported, although the latency period (time from recruitment to delivery) was increased in the group receiving the combination of tocolytics.For other combinations of tocolytic agents, primary outcomes were rarely reported and for secondary outcomes results did not demonstrate differences between groups.
Authors' conclusions: It is unclear whether a combination of tocolytic drugs for preterm labour is more advantageous for women and/or newborns due to a lack of large, well-designed trials including the outcomes of interest. There are no trials of combination regimens using widely used tocolytic agents, such as calcium channel blockers (nifedipine) and/or oxytocin receptor antagonists (atosiban). Further trials are needed before specific conclusions on use of combination tocolytic therapy for preterm labour can be made.
Conflict of interest statement
Therese Dowswell and Helen West were paid for work on this review from a grant to their institution (University of Liverpool) from the World Health Organization.
Figures






























Update of
- doi: 10.1002/14651858.CD006169
Similar articles
-
Calcium channel blockers for inhibiting preterm labour and birth.Cochrane Database Syst Rev. 2014 Jun 5;2014(6):CD002255. doi: 10.1002/14651858.CD002255.pub2. Cochrane Database Syst Rev. 2014. PMID: 24901312 Free PMC article.
-
Oxytocin receptor antagonists for inhibiting preterm labour.Cochrane Database Syst Rev. 2014 Jun 6;2014(6):CD004452. doi: 10.1002/14651858.CD004452.pub3. Cochrane Database Syst Rev. 2014. PMID: 24903678 Free PMC article.
-
Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour.Cochrane Database Syst Rev. 2015 Dec 14;2015(12):CD011200. doi: 10.1002/14651858.CD011200.pub2. Cochrane Database Syst Rev. 2015. PMID: 26662716 Free PMC article.
-
Acute tocolysis for uterine tachysystole or suspected fetal distress.Cochrane Database Syst Rev. 2018 Jul 4;7(7):CD009770. doi: 10.1002/14651858.CD009770.pub2. Cochrane Database Syst Rev. 2018. PMID: 29971813 Free PMC article.
-
Cyclo-oxygenase (COX) inhibitors for treating preterm labour.Cochrane Database Syst Rev. 2015 Jun 5;2015(6):CD001992. doi: 10.1002/14651858.CD001992.pub3. Cochrane Database Syst Rev. 2015. PMID: 26042617 Free PMC article.
Cited by
-
Frontiers in the Etiology and Treatment of Preterm Premature Rupture of Membrane: From Molecular Mechanisms to Innovative Therapeutic Strategies.Reprod Sci. 2024 Apr;31(4):917-931. doi: 10.1007/s43032-023-01411-9. Epub 2023 Nov 21. Reprod Sci. 2024. PMID: 37989803 Review.
-
Tocolysis among Women with Preterm Birth: Associated Factors and Outcomes from a Multicenter Study in Brazil.Rev Bras Ginecol Obstet. 2018 Apr;40(4):171-179. doi: 10.1055/s-0038-1642025. Epub 2018 May 10. Rev Bras Ginecol Obstet. 2018. PMID: 29747211 Free PMC article.
-
Altered uterine contractility in response to β-adrenoceptor agonists in ovarian cancer.J Physiol Sci. 2017 Nov;67(6):711-722. doi: 10.1007/s12576-016-0500-1. Epub 2016 Nov 12. J Physiol Sci. 2017. PMID: 27838886 Free PMC article.
-
Combined uterorelaxant effect of magnesium sulfate and terbutaline: Studies on late pregnant rat uteri in vitro and in vivo.Acta Obstet Gynecol Scand. 2023 Apr;102(4):457-464. doi: 10.1111/aogs.14532. Epub 2023 Feb 19. Acta Obstet Gynecol Scand. 2023. PMID: 36808376 Free PMC article.
-
Prevention and Therapy of Preterm Birth. Guideline of the DGGG, OEGGG and SGGG (S2k Level, AWMF Registry Number 015/025, February 2019) - Part 2 with Recommendations on the Tertiary Prevention of Preterm Birth and the Management of Preterm Premature Rupture of Membranes.Geburtshilfe Frauenheilkd. 2019 Aug;79(8):813-833. doi: 10.1055/a-0903-2735. Epub 2019 Aug 12. Geburtshilfe Frauenheilkd. 2019. PMID: 31423017 Free PMC article.
References
References to studies included in this review
Ally 1992 {published data only}
-
- Ally K, Nicolas A, Thoumsin H, Lambotte R. Magnesium gluconate and intravenous tocolysis with ritodrine. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1992;21:370‐4. - PubMed
Arikan 2011 {published data only}
-
- Arikan I, Barut A, Harma M, Harma IM. Effect of progesterone as a tocolytic and in maintenance therapy during preterm labor. Gynecologic and Obstetric Investigation 2011;72(4):269‐73. - PubMed
Castillo 1988 {published data only}
-
- Castillo JM, Alonso J, Hernandez‐Garcia JM, Sancho B, Martinez V. Study of biochemical and biophysical modifications produced on pregnant women treated in threatened of premature labor with ritodrine and indometacine. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988:20.
Ferguson 1984 {published data only}
-
- Ferguson JE, Hensleigh PA, Kredenster D. Adjunctive use of magnesium sulfate with ritodrine for preterm labor tocolysis. American Journal of Obstetrics and Gynecology 1984;148(2):166‐71. - PubMed
-
- Ferguson JE, Holbrook H, Stevenson DK, Hensleigh PA, Kredentser D. Adjunctive magnesium sulphate infusion does not alter metabolic changes associated with ritodrine tocolysis. American Journal of Obstetrics and Gynecology 1987;156:103‐7. - PubMed
Francioli 1988 {published data only}
-
- Francioli M, Meuron A. Usefulness of the addition of aspartate magnesium hydrochloride via intravenous route to beta mimetics in the treatment of threatened preterm labor. Revue Medicale de la Suisse Romande 1988;108:283‐9. - PubMed
Gamissans 1982 {published data only}
-
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. European Journal of Obstetrics and Gynecology and Reproductive Biology 1978;8:123‐8. - PubMed
-
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. Proceedings of the 6th European Congress of Perinatal Medicine, August 29th‐September 1st. Vienna, Austria, 1978. - PubMed
-
- Gamissans O, Cararach V, Serra J. The role of prostaglandin‐inhibitors, beta‐adrenergic drugs and glucocorticoids in the management of threatened preterm labor. Beta‐mimetic Drugs in Obstetrics and Perinatology. 3rd Symposium on Beta‐mimetic Drugs, Aagen, November 1980. Thieme‐Stratton Inc, 1982:71‐84.
Hatjis 1987 {published data only}
-
- Hatjis CG, Swain M, Nelson LH, Meis PJ, Ernest JM. Efficacy of combined administration of magnesium sulfate and ritodrine in the treatment of premature labor. Obstetrics and Gynecology 1987;69:317‐22. - PubMed
Lauterbach 2012 {published data only}
-
- Lauterbach R, Rytlewski K, Pawlik D, Hurkala J, Wojtowicz A, Breborowicz G, et al. Effect of Pentoxifylline, administered in preterm labour, on the foetal‐placental circulation and neonatal outcome: a randomized, prospective pilot study. Basic and Clinical Pharmacology and Toxicology 2012;110(4):342‐6. - PubMed
Rios‐Anez 2001 {published data only}
-
- Rios‐Anez R, Santos‐Luque M, Noguera ME. Use of an antagonist of the prostaglandins associated to a b‐sympathomimetic agent in the treatment of pre‐term labour [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):165.
Ross 1983 {published data only}
-
- Ross MG, Nicolls E, Stubblefield PG, Kitzmiller JL. Intravenous terbutaline and simultaneous beta‐blockade for advanced premature labor. American Journal of Obstetrics and Gynecology 1983;147:897‐902. - PubMed
Schauf 2005 {published data only}
-
- Schauf B, Becker S, Abele H, Klever T, Wallwiener D, Aydeniz B. Effect of magnesium on red blood cell deformability in pregnancy. Hypertension in Pregnancy 2005;24(1):17‐27. - PubMed
References to studies excluded from this review
Bedoya 1972 {published data only}
-
- Bedoya JM. Use of orciprenaline in the treatment of threatened premature labour. Proceedings of International Symposium on the Treatment of Fetal Risks; 1972; Baden, Austria. 1972:27‐9.
Caballero 1979 {published data only}
-
- Caballero A, Tejerina A, Dominguez A, Nava JM, Caballero A Jr. Indomethacine alone or associated to ritodrine in the prevention of premature labour [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 Oct 26‐31;Tokyo, Japan. 1979:300.
Dunlop 1986 {published data only}
-
- Dunlop PDM, Crowley PA, Lamont RF, Hawkins DF. Preterm ruptured membranes, no contractions. Journal of Obstetrics and Gynaecology 1986;7:92‐6.
Herzog 1999 {published data only}
-
- Herzog S, Cunze T, Martin M, Osmers R, Gleiter C, Kuhn W. Pulsatile vs continuous parenteral tocolysis: comparison of side effects. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;85:199‐204. - PubMed
-
- Herzog S, Cunze T, Osmers R, Kuhn W. Comparative study of maternal side effects of various forms of intravenous therapy with fenoterol in premature labor. Gynakologisch Geburtshilfliche Rundschau 1995;35(1):73‐9. - PubMed
How 2006 {published data only}
-
- How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai BM, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. American Journal of Obstetrics and Gynecology 2006;194(4):976‐81. - PubMed
Ieda 1991 {published data only}
-
- Ieda K, Sasaki J, Mizuno S, Mimura S, Suzuki C, Miyazaki T, et al. The clinical effects of maternally administered magnesium sulfate on the neonate. Journal of Perinatal Medicine 1991;Suppl 2:225.
Illia 1993 {published data only}
-
- Illia R, Diego I, Solana C. Threatened preterm labour. Evaluation of perinatals results in patients treated with betamimetics only and associated with indometacin [Amenaza de parto pretérmino. Evaluación de resultados perinatales de pacientes tratadas con betamiméticos solos y asociados a indometacina]. Tokoginecologia Practica 1993;52:383‐7.
Katz 1983 {published data only}
-
- Katz Z, Lancet M, Yemini M, Mogilner BM, Feigl A, Ben‐Hur H. Treatment of premature labor contractions with combined ritodrine and indomethacine. International Journal of Gynecology & Obstetrics 1983;21:337‐42. - PubMed
Kawagoe 2011 {published data only}
Morales 2013 {published data only}
-
- Morales CV. Utility of tocolytic therapy for maintenance tocolysis in the management of threatened preterm delivery (UTM/2012). ClinicalTrials.gov (accessed 21 May 2013) 2013.
Ogburn 1985 {published data only}
-
- Ogburn PL Jr, Hansen CA, Williams PP, Butler JC Jr, Joseph MS, Julian TM. Magnesium sulfate and beta‐mimetic dual‐agent tocolysis in preterm labor after single‐agent failure. Journal of Reproductive Medicine 1985;30(8):583‐7. - PubMed
Reynolds 1978 {published data only}
-
- Reynolds JW. A comparison of salbutamol and ethanol in the treatment of premature labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1978;18:107‐9.
Richter 1975 {published data only}
-
- Richter R, Hammacher K, Hinselmann M. Prophylaxis of threatened premature labour. Clinical results in a prospective comparative study with ritodrine and buphenine. Gynakologische Rundschau 1975;15:58‐9. - PubMed
Richter 1979 {published data only}
-
- Richter R, Hinselmann MJ. The treatment of threatened premature labor by betamimetic drugs: a comparison of fenoterol and ritodrine. Obstetrics & Gynecology 1979;53:81‐7. - PubMed
Spearing 1979 {published data only}
-
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. - PubMed
Wischnik 1989 {published data only}
-
- Wischnik A, Hettenbach A, Schmidt R, Zieger W, Hug G, Melchert F. The effect of magnesium sulfate on volume balance in tocolysis with the beta mimetic agent fenoterol. Gynakologische Rundschau 1989;29 Suppl 2:188‐91. - PubMed
Additional references
Anotayanonth 2004
Blencowe 2012
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since1990 for selected countries: a systematic analysis and implications. Lancet 2012;9(379):2162‐72. - PubMed
Born Too Soon Report 2012
-
- March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon Report: The Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization, 2012.
Boyle 2011
Bryce 2005
-
- Bryce J, Boschi‐Pinto C, Shibuya K, Black RE, for the WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet 2005;365(9465):1147‐52. - PubMed
Caritis 2005
-
- Caritis S. Adverse effects of tocolytic therapy. BJOG: an international journal of obstetrics and gynaecology 2005;112(Suppl 1):74‐8. - PubMed
Crowther 2002
Davidoff 2006
-
- Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Seminars in Perinatology 2006;30(5):313. - PubMed
Duckitt 2002
Ekeus 2010
-
- Ekeus C, Lindström K, Lindblad F, Rasmussen F, Hjern A. Preterm birth, social disadvantage, and cognitive competence in Swedish 18‐ to 19‐year‐old men. Paediatrics 2010;125:e67‐e73. - PubMed
Flenady 2014
Flenady 2014a
Gaunekar 2004
Goldenberg 2008
Gáspár 2013
Hagberg 2001
-
- Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991‐94. Acta Paediatrica 2001;90(3):271‐7. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katz 2013
King 2005
Lawn 2009
-
- Lawn JE, Kerber K, Enweronu‐Laryea C, Cousens S. 3.6 million neonatal deaths—what is progressing and what is not?. Seminars in Perinatology 2010;34(6):371‐86. - PubMed
Marlow 2005
-
- Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. New England Journal of Medicine 2005;352(1):9‐19. - PubMed
Noble 2009
-
- Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144:S11‐S19. - PubMed
O'Connor 2007
-
- O'Connor AR, Wilson CM, Fielder AR. Ophthalmological problems associated with preterm birth. Eye (London) 2007;21(10):1254‐60. - PubMed
Plunkett 2008
-
- Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Annals of Medicine 2008;40:167‐95. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
Talge 2010
-
- Talge N, Holzman C, Wang J, Lucia V. Late‐preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics 2010;126(6):1124‐31. - PubMed
Teune 2011
-
- Teune MJ, Bakhuizen S, Gyamfi Bannerman C, Opmeer BC, Kaam AH, Wassenaer AG, et al. A systematic review of severe morbidity in infants born late preterm. American Journal of Obstetrics and Gynecology 2011;205(4):374.e1‐374.e9. - PubMed
van Baar 2009
-
- Baar ALA, Vermaas JJ, Knots EE, Kleine MJKM, Soons PP. Functioning at school age of moderately preterm children born at 32 to 36 weeks' gestational age. Paediatrics 2009;124(1):251‐7. - PubMed
Wray 2003
-
- Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir‐Bishty E, et al. Calcium signalling and uterine contractility. Journal of the Society for Gynecologic Investigation 2003;10:252‐64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

