Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway
- PMID: 25012175
- PMCID: PMC4310644
- DOI: 10.1681/ASN.2013070703
Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway
Abstract
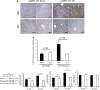
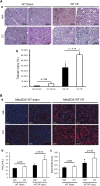
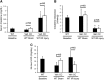
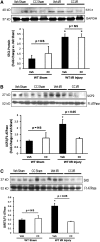
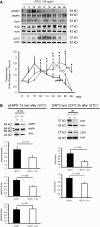
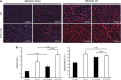
AKI is associated with increased morbidity, mortality, and cost of care, and therapeutic options remain limited. Reactive oxygen species are critical for the genesis of ischemic AKI. Stanniocalcin-1 (STC1) suppresses superoxide generation through induction of uncoupling proteins (UCPs), and transgenic overexpression of STC1 inhibits reactive oxygen species and protects from ischemia/reperfusion (I/R) kidney injury. Our observations revealed high AMP-activated protein kinase (AMPK) activity in STC1 transgenic kidneys relative to wild-type (WT) kidneys; thus, we hypothesized that STC1 protects from I/R kidney injury through activation of AMPK. Baseline activity of AMPK in the kidney correlated with the expression of STCs, such that the highest activity was observed in STC1 transgenic mice followed (in decreasing order) by WT, STC1 knockout, and STC1/STC2 double-knockout mice. I/R in WT kidneys increased AMPK activity and the expression of STC1, UCP2, and sirtuin 3. Inhibition of AMPK by administration of compound C before I/R abolished the activation of AMPK, diminished the expression of UCP2 and sirtuin 3, and aggravated kidney injury but did not affect STC1 expression. Treatment of cultured HEK cells with recombinant STC1 activated AMPK and increased the expression of UCP2 and sirtuin 3, and concomitant treatment with compound C abolished these responses. STC1 knockout mice displayed high susceptibility to I/R, whereas pretreatment of STC1 transgenic mice with compound C restored the susceptibility to I/R kidney injury. These data suggest that STC1 is important for activation of AMPK in the kidney, which mediates STC1-induced expression of UCP2 and sirtuin 3 and protection from I/R.
Keywords: ischemia-reperfusion; mitochondria; protection; reactive oxygen species; renal; renal injury; renal morphology.
Copyright © 2015 by the American Society of Nephrology.
Figures



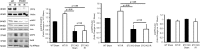

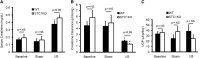

References
-
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 - PubMed
-
- Carpenter CB: Long-term failure of renal transplants: Adding insult to injury. Kidney Int Suppl 50: S40–S44, 1995 - PubMed
-
- Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV: Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem 278: 27256–27266, 2003 - PubMed
-
- Gueler F, Gwinner W, Schwarz A, Haller H: Long-term effects of acute ischemia and reperfusion injury. Kidney Int 66: 523–527, 2004 - PubMed
-
- Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM: The “injury response”: A concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 29: 79–81, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

