Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules
- PMID: 25013173
- PMCID: PMC4117795
- DOI: 10.1093/nar/gku582
Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules
Abstract
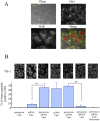
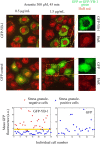
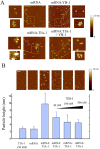
The sequence of events leading to stress granule assembly in stressed cells remains elusive. We show here, using isotope labeling and ion microprobe, that proportionally more RNA than proteins are present in stress granules than in surrounding cytoplasm. We further demonstrate that the delivery of single strand polynucleotides, mRNA and ssDNA, to the cytoplasm can trigger stress granule assembly. On the other hand, increasing the cytoplasmic level of mRNA-binding proteins like YB-1 can directly prevent the aggregation of mRNA by forming isolated mRNPs, as evidenced by atomic force microscopy. Interestingly, we also discovered that enucleated cells do form stress granules, demonstrating that the translocation to the cytoplasm of nuclear prion-like RNA-binding proteins like TIA-1 is dispensable for stress granule assembly. The results lead to an alternative view on stress granule formation based on the following sequence of events: after the massive dissociation of polysomes during stress, mRNA-stabilizing proteins like YB-1 are outnumbered by the burst of nonpolysomal mRNA. mRNA freed of ribosomes thus becomes accessible to mRNA-binding aggregation-prone proteins or misfolded proteins, which induces stress granule formation. Within the frame of this model, the shuttling of nuclear mRNA-stabilizing proteins to the cytoplasm could dissociate stress granules or prevent their assembly.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

