Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone
- PMID: 25013998
- PMCID: PMC4399272
- DOI: 10.1210/jc.2014-1843
Changes in plasma ACTH levels and corticotroph tumor size in patients with Cushing's disease during long-term treatment with the glucocorticoid receptor antagonist mifepristone
Abstract
Context: Pituitary effects of long-term therapy with mifepristone, a glucocorticoid receptor antagonist, in Cushing's disease (CD) patients are not well understood.
Objective: Our objective was to report changes in ACTH and pituitary magnetic resonance imaging (MRI) findings during long-term use of mifepristone in CD patients.
Design and setting: The Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing's Syndrome (SEISMIC) was a 24-week, open-label study of mifepristone, and its long-term extension (LTE) is a multicenter U.S. study.
Patients: Forty-three CD patients (mean age 45.3 years) were enrolled in SEISMIC with 27 continuing into the LTE study.
Interventions: Mifepristone (300-1200 mg) was administered once daily.
Main outcome measures: ACTH and pituitary MRI were assessed at baseline and at regular intervals during treatment.
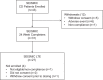
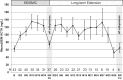
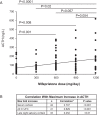
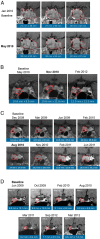
Results: A ≥2-fold increase in ACTH was observed in 72% of patients treated for a median duration of 11.3 months. The mean peak increase in ACTH was 2.76 ± 1.65-fold during SEISMIC, and mean ACTH concentrations remained stable during the LTE. ACTH was directly correlated with mifepristone dose and declined to near baseline levels after mifepristone discontinuation. Tumor regressed in 2 patients and progressed in 3 patients with macroadenomas. An additional microadenoma was identified after 25 months of treatment after a baseline tumor-negative MRI.
Conclusions: In the largest prospective study to date, long-term mifepristone treatment increased ACTH in approximately two-thirds of patients with CD. ACTH elevations were observed within the first few weeks of treatment, were dose-dependent, and generally remained stable over time. Corticotroph tumor progression and regression may occur over time, but patients may have significant increases in ACTH levels without evidence of tumor growth.
Trial registration: ClinicalTrials.gov NCT00569582 NCT00936741.
Figures
Similar articles
-
Genetically engineered human pituitary corticotroph tumor organoids exhibit divergent responses to glucocorticoid receptor modulators.Transl Res. 2023 Jun;256:56-72. doi: 10.1016/j.trsl.2023.01.002. Epub 2023 Jan 12. Transl Res. 2023. PMID: 36640905 Free PMC article.
-
Biochemical assessment of Cushing's disease in patients with corticotroph macroadenomas.J Clin Endocrinol Metab. 1998 May;83(5):1619-23. doi: 10.1210/jcem.83.5.4845. J Clin Endocrinol Metab. 1998. PMID: 9589666
-
Sustained weight loss in patients treated with mifepristone for Cushing's syndrome: a follow-up analysis of the SEISMIC study and long-term extension.BMC Endocr Disord. 2015 Oct 27;15:63. doi: 10.1186/s12902-015-0059-5. BMC Endocr Disord. 2015. PMID: 26507877 Free PMC article.
-
The Treatment of Cushing's Disease.Endocr Rev. 2015 Aug;36(4):385-486. doi: 10.1210/er.2013-1048. Epub 2015 Jun 11. Endocr Rev. 2015. PMID: 26067718 Free PMC article. Review.
-
Somatostatin and somatostatin receptors in Cushing's disease.Mol Cell Endocrinol. 2008 May 14;286(1-2):199-205. doi: 10.1016/j.mce.2007.10.015. Epub 2007 Nov 22. Mol Cell Endocrinol. 2008. PMID: 18221833 Review.
Cited by
-
Cushing's Disease.J Clin Med. 2019 Nov 12;8(11):1951. doi: 10.3390/jcm8111951. J Clin Med. 2019. PMID: 31726770 Free PMC article. Review.
-
Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study.Front Endocrinol (Lausanne). 2021 Jul 14;12:662865. doi: 10.3389/fendo.2021.662865. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34335465 Free PMC article. Clinical Trial.
-
No Postoperative Adrenal Insufficiency in a Patient with Unilateral Cortisol-Secreting Adenomas Treated with Mifepristone Before Surgery.Clin Med Insights Endocrinol Diabetes. 2016 Jul 26;9:31-6. doi: 10.4137/CMED.S39997. eCollection 2016. Clin Med Insights Endocrinol Diabetes. 2016. PMID: 27486349 Free PMC article.
-
Management of Nelson's Syndrome.Medicina (Kaunas). 2022 Nov 2;58(11):1580. doi: 10.3390/medicina58111580. Medicina (Kaunas). 2022. PMID: 36363537 Free PMC article. Review.
-
Advancing Treatment of Pituitary Adenomas through Targeted Molecular Therapies: The Acromegaly and Cushing Disease Paradigms.Front Surg. 2016 Jul 28;3:45. doi: 10.3389/fsurg.2016.00045. eCollection 2016. Front Surg. 2016. PMID: 27517036 Free PMC article. Review.
References
-
- Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96:632–642. - PubMed
-
- Dekkers OM, Horváth-Puhó E, Jørgensen JO, et al. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98:2277–2284. - PubMed
-
- Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing's disease: the need for long-term surveillance. Clin Endocrinol (Oxf). 2005;63:549–559. - PubMed
-
- Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab. 2011;96:1992–2003. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

