Current and emerging treatment options for fecal incontinence
- PMID: 25014235
- PMCID: PMC4166012
- DOI: 10.1097/MCG.0000000000000180
Current and emerging treatment options for fecal incontinence
Abstract
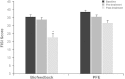
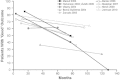
Fecal incontinence (FI) is a multifactorial disorder that imposes considerable social and economic burdens. The aim of this article is to provide an overview of current and emerging treatment options for FI. A MEDLINE search was conducted for English-language articles related to FI prevalence, etiology, diagnosis, and treatment published from January 1, 1990 through June 1, 2013. The search was extended to unpublished trials on ClinicalTrials.gov and relevant publications cited in included articles. Conservative approaches, including dietary modifications, medications, muscle-strengthening exercises, and biofeedback, have been shown to provide short-term benefits. Transcutaneous electrical stimulation was considered ineffective in a randomized clinical trial. Unlike initial studies, sacral nerve stimulation has shown reasonable short-term effectiveness and some complications. Dynamic graciloplasty and artificial sphincter and bowel devices lack randomized controlled trials and have shown inconsistent results and high rates of explantation. Of injectable bulking agents, dextranomer microspheres in non-animal stabilized hyaluronic acid (NASHA Dx) has shown significant improvement in incontinence scores and frequency of incontinence episodes, with generally mild adverse effects. For the treatment of FI, conservative measures and biofeedback therapy are modestly effective. When conservative therapies are ineffective, invasive procedures, including sacral nerve stimulation, may be considered, but they are associated with complications and lack randomized, controlled trials. Bulking agents may be an appropriate alternative therapy to consider before more aggressive therapies in patients who fail conservative therapies.
Conflict of interest statement
S.S.C.R. is a member of the Data Monitoring Board for American Medical Systems Inc., but has no financial interests with any other fecal incontinence product manufacturer. Salix Pharmaceuticals did not contribute to content development of the manuscript.
Figures


References
-
- Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586–598 - PubMed
-
- US Census Bureau. People QuickFacts, USA. 2013. Available at: http://quickfacts.census.gov/qfd/states/00000.html. Accessed July 9, 2013
-
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in US adults, 2005-2010. Clin Gastroenterol Hepatol. 2013636–643 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

