The role of mechanical forces in tumor growth and therapy
- PMID: 25014786
- PMCID: PMC4109025
- DOI: 10.1146/annurev-bioeng-071813-105259
The role of mechanical forces in tumor growth and therapy
Abstract
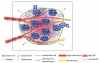
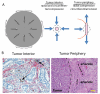
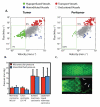
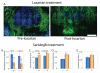
Tumors generate physical forces during growth and progression. These physical forces are able to compress blood and lymphatic vessels, reducing perfusion rates and creating hypoxia. When exerted directly on cancer cells, they can increase cells' invasive and metastatic potential. Tumor vessels-while nourishing the tumor-are usually leaky and tortuous, which further decreases perfusion. Hypoperfusion and hypoxia contribute to immune evasion, promote malignant progression and metastasis, and reduce the efficacy of a number of therapies, including radiation. In parallel, vessel leakiness together with vessel compression causes a uniformly elevated interstitial fluid pressure that hinders delivery of blood-borne therapeutic agents, lowering the efficacy of chemo- and nanotherapies. In addition, shear stresses exerted by flowing blood and interstitial fluid modulate the behavior of cancer and a variety of host cells. Taming these physical forces can improve therapeutic outcomes in many cancers.
Keywords: solid stress; stress alleviation; tumor microenvironment; tumor perfusion; vascular hyperpermeability; vascular normalization; vessel compression.
Figures


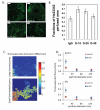
References
-
- Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–83. - PubMed
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA073479/CA/NCI NIH HHS/United States
- R01 CA126642/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- T32-CA0734/CA/NCI NIH HHS/United States
- R01 CA098706/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- P41 EB015903/EB/NIBIB NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
- R01-CA098706/CA/NCI NIH HHS/United States
- 336839/ERC_/European Research Council/International
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

