Regulation of the pentose phosphate pathway in cancer
- PMID: 25015087
- PMCID: PMC4112277
- DOI: 10.1007/s13238-014-0082-8
Regulation of the pentose phosphate pathway in cancer
Abstract
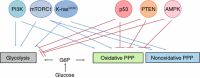
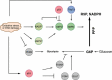
Energy metabolism is significantly reprogrammed in many human cancers, and these alterations confer many advantages to cancer cells, including the promotion of biosynthesis, ATP generation, detoxification and support of rapid proliferation. The pentose phosphate pathway (PPP) is a major pathway for glucose catabolism. The PPP directs glucose flux to its oxidative branch and produces a reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), an essential reductant in anabolic processes. It has become clear that the PPP plays a critical role in regulating cancer cell growth by supplying cells with not only ribose-5-phosphate but also NADPH for detoxification of intracellular reactive oxygen species, reductive biosynthesis and ribose biogenesis. Thus, alteration of the PPP contributes directly to cell proliferation, survival and senescence. Furthermore, recent studies have shown that the PPP is regulated oncogenically and/or metabolically by numerous factors, including tumor suppressors, oncoproteins and intracellular metabolites. Dysregulation of PPP flux dramatically impacts cancer growth and survival. Therefore, a better understanding of how the PPP is reprogrammed and the mechanism underlying the balance between glycolysis and PPP flux in cancer will be valuable in developing therapeutic strategies targeting this pathway.
Figures


References
-
- Amelio I, Markert EK, Rufini A, Antonov AV, Sayan BS, Tucci P, Agostini M, Mineo TC, Levine AJ, Melino G (2013) p73 regulates serine biosynthesis in cancer. Oncogene. doi:10.1038/onc.2013.456 - PubMed
-
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

