Maternal vaccination: moving the science forward
- PMID: 25015234
- PMCID: PMC4255605
- DOI: 10.1093/humupd/dmu041
Maternal vaccination: moving the science forward
Abstract
Background: Infections remain one of the leading causes of morbidity in pregnant women and newborns, with vaccine-preventable infections contributing significantly to the burden of disease. In the past decade, maternal vaccination has emerged as a promising public health strategy to prevent and combat maternal, fetal and neonatal infections. Despite a number of universally recommended maternal vaccines, the development and evaluation of safe and effective maternal vaccines and their wide acceptance are hampered by the lack of thorough understanding of the efficacy and safety in the pregnant women and the offspring.
Methods: An outline was synthesized based on the current status and major gaps in the knowledge of maternal vaccination. A systematic literature search in PUBMED was undertaken using the key words in each section title of the outline to retrieve articles relevant to pregnancy. Articles cited were selected based on relevance and quality. On the basis of the reviewed information, a perspective on the future directions of maternal vaccination research was formulated.
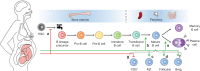
Results: Maternal vaccination can generate active immune protection in the mother and elicit systemic immunoglobulin G (IgG) and mucosal IgG, IgA and IgM responses to confer neonatal protection. The maternal immune system undergoes significant modulation during pregnancy, which influences responsiveness to vaccines. Significant gaps exist in our knowledge of the efficacy and safety of maternal vaccines, and no maternal vaccines against a large number of old and emerging pathogens are available. Public acceptance of maternal vaccination has been low.
Conclusions: To tackle the scientific challenges of maternal vaccination and to provide the public with informed vaccination choices, scientists and clinicians in different disciplines must work closely and have a mechanistic understanding of the systemic, reproductive and mammary mucosal immune responses to vaccines. The use of animal models should be coupled with human studies in an iterative manner for maternal vaccine experimentation, evaluation and optimization. Systems biology approaches should be adopted to improve the speed, accuracy and safety of maternal vaccine targeting.
Keywords: animal model; antibody; immunology; pregnancy; vaccine.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
B cell responses in pregnancy and vaccine efficacy.Hum Reprod Update. 2015 May-Jun;21(3):407-8. doi: 10.1093/humupd/dmv009. Epub 2015 Feb 23. Hum Reprod Update. 2015. PMID: 25712339 No abstract available.
-
Reply: Maternal vaccination: moving the science forward.Hum Reprod Update. 2015 May-Jun;21(3):408-9. doi: 10.1093/humupd/dmv010. Epub 2015 Feb 23. Hum Reprod Update. 2015. PMID: 25712340 Free PMC article. No abstract available.
References
-
- Abram M, Schluter D, Vuckovic D, Wraber B, Doric M, Deckert M. Murine model of pregnancy-associated Listeria monocytogenes infection. FEMS Immunol Med Microbiol. 2003;35:177–182. - PubMed
-
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. - PubMed
-
- Ait-Azzouzene D, Gendron MC, Houdayer M, Langkopf A, Burki K, Nemazee D, Kanellopoulos-Langevin C. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J Immunol. 1998;161:2677–2683. - PubMed
-
- Ait-Azzouzene D, Caucheteux S, Tchang F, Wantyghem J, Moutier R, Langkopf A, Gendron MC, Kanellopoulos-Langevin C. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol Reprod. 2001;65:337–344. - PubMed
-
- Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

