Mechanisms of activation of mouse and human enteroendocrine cells by nutrients
- PMID: 25015642
- PMCID: PMC4392230
- DOI: 10.1136/gutjnl-2014-306834
Mechanisms of activation of mouse and human enteroendocrine cells by nutrients
Abstract
Objective: Inhibition of food intake and glucose homeostasis are both promoted when nutrients stimulate enteroendocrine cells (EEC) to release gut hormones. Several specific nutrient receptors may be located on EEC that respond to dietary sugars, amino acids and fatty acids. Bypass surgery for obesity and type II diabetes works by shunting nutrients to the distal gut, where it increases activation of nutrient receptors and mediator release, but cellular mechanisms of activation are largely unknown. We determined which nutrient receptors are expressed in which gut regions and in which cells in mouse and human, how they are associated with different types of EEC, how they are activated leading to hormone and 5-HT release.
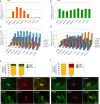
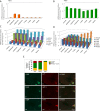
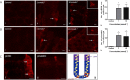
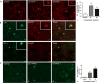
Design and results: mRNA expression of 17 nutrient receptors and EEC mediators was assessed by quantitative PCR and found throughout mouse and human gut epithelium. Many species similarities emerged, in particular the dense expression of several receptors in the distal gut. Immunolabelling showed specific colocalisation of receptors with EEC mediators PYY and GLP-1 (L-cells) or 5-HT (enterochromaffin cells). We exposed isolated proximal colonic mucosa to specific nutrients, which recruited signalling pathways within specific EEC extracellular receptor-regulated kinase (p-ERK) and calmodulin kinase II (pCAMKII), as shown by subsequent immunolabelling, and activated release of these mediators. Aromatic amino acids activated both pathways in mouse, but in humans they induced only pCAMKII, which was colocalised mainly with 5-HT expression. Activation was pertussis toxin-sensitive. Fatty acid (C12) potently activated p-ERK in human in all EEC types and evoked potent release of all three mediators.
Conclusions: Specific nutrient receptors associate with distinct activation pathways within EEC. These may provide discrete, complementary pharmacological targets for intervention in obesity and type II diabetes.
Keywords: Gut Hormones; Obesity.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
