Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema
- PMID: 25017446
- PMCID: PMC4165711
- DOI: 10.1016/j.neuroimage.2014.06.064
Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema
Abstract
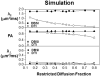
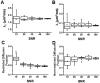
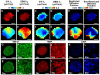
The effect of extra-fiber structural and pathological components confounding diffusion tensor imaging (DTI) computation was quantitatively investigated using data generated by both Monte-Carlo simulations and tissue phantoms. Increased extent of vasogenic edema, by addition of various amount of gel to fixed normal mouse trigeminal nerves or by increasing non-restricted isotropic diffusion tensor components in Monte-Carlo simulations, significantly decreased fractional anisotropy (FA) and increased radial diffusivity, while less significantly increased axial diffusivity derived by DTI. Increased cellularity, mimicked by graded increase of the restricted isotropic diffusion tensor component in Monte-Carlo simulations, significantly decreased FA and axial diffusivity with limited impact on radial diffusivity derived by DTI. The MC simulation and tissue phantom data were also analyzed by the recently developed diffusion basis spectrum imaging (DBSI) to simultaneously distinguish and quantify the axon/myelin integrity and extra-fiber diffusion components. Results showed that increased cellularity or vasogenic edema did not affect the DBSI-derived fiber FA, axial or radial diffusivity. Importantly, the extent of extra-fiber cellularity and edema estimated by DBSI correlated with experimentally added gel and Monte-Carlo simulations. We also examined the feasibility of applying 25-direction diffusion encoding scheme for DBSI analysis on coherent white matter tracts. Results from both phantom experiments and simulations suggested that the 25-direction diffusion scheme provided comparable DBSI estimation of both fiber diffusion parameters and extra-fiber cellularity/edema extent as those by 99-direction scheme. An in vivo 25-direction DBSI analysis was performed on experimental autoimmune encephalomyelitis (EAE, an animal model of human multiple sclerosis) optic nerve as an example to examine the validity of derived DBSI parameters with post-imaging immunohistochemistry verification. Results support that in vivo DBSI using 25-direction diffusion scheme correctly reflect the underlying axonal injury, demyelination, and inflammation of optic nerves in EAE mice.
Keywords: Diffusion basis spectrum imaging; Diffusion tensor imaging; Experimental autoimmune encephalomyelitis (EAE); Immunohistochemistry (IHC); Inflammation; Magnetic resonance imaging; Monte-Carlo simulation; Multiple tensor model; Restricted diffusion; White matter injury.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


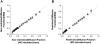

References
-
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, Dyrby TB. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–1389. - PubMed
-
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–1139. - PubMed
-
- Anderson AW, Xie J, Pizzonia J, Bronen RA, Spencer DD, Gore JC. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn Reson Imaging. 2000;18:689–695. - PubMed
-
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage. 2005;27:48–58. - PubMed
-
- Assaf Y, Ben-Bashat D, Chapman J, Peled S, Biton IE, Kafri M, Segev Y, Hendler T, Korczyn AD, Graif M, Cohen Y. High b-value q-space analyzed diffusion-weighted MRI: application to multiple sclerosis. Magn Reson Med. 2002;47:115–126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

