Antiviral drug allergy
- PMID: 25017682
- PMCID: PMC4140860
- DOI: 10.1016/j.iac.2014.04.011
Antiviral drug allergy
Abstract
Antiviral drugs used to treat HIV and hepatitis C are common causes of delayed drug hypersensitivities for which many of the more severe reactions have been recently shown to be immunogenetically mediated such as abacavir hypersensitivity where HLA-B(∗)57:01 is now used routinely as a screening test to exclude patients carrying this allele from abacavir prescription. Most antiviral drug allergies consist of mild to moderate delayed rash without other serious features (eg, fever, mucosal involvement, blistering rash, organ impairment. In these cases treatment can be continued with careful observation and symptomatic management and the discontinuation rate is low.
Keywords: Abacavir; Altered peptide repertoire; Antiretroviral; Human leukocyte antigen; Major histocompatibility complex; Nevirapine; Telaprevir pharmacogenomics.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures/conflict of interest: none related to the content of this article
Figures
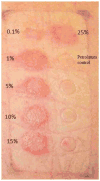
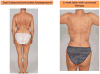




References
-
- Vilar FJ, Naisbitt DJ, Park BK, et al. Mechanisms of drug hypersensitivity in HIV-infected patients: the role of the immune system. J HIV Ther. 2003;8:42–47. - PubMed
-
- Cacoub P, Bourliere M, Lubbe J, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455–463. - PubMed
-
- Phillips E, Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol. 2007;7:324–330. - PubMed
-
- Clay PG. The abacavir hypersensitivity reaction: a review. Clin Ther. 2002;24:1502–1514. - PubMed
-
- Vitezica ZG, Milpied B, Lonjou C, et al. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

