Pattern recognition receptors and central nervous system repair
- PMID: 25017883
- PMCID: PMC4974939
- DOI: 10.1016/j.expneurol.2014.01.001
Pattern recognition receptors and central nervous system repair
Abstract
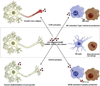
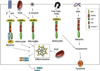
Pattern recognition receptors (PRRs) are part of the innate immune response and were originally discovered for their role in recognizing pathogens by ligating specific pathogen associated molecular patterns (PAMPs) expressed by microbes. Now the role of PRRs in sterile inflammation is also appreciated, responding to endogenous stimuli referred to as "damage associated molecular patterns" (DAMPs) instead of PAMPs. The main families of PRRs include Toll-like receptors (TLRs), Nod-like receptors (NLRs), RIG-like receptors (RLRs), AIM2-like receptors (ALRs), and C-type lectin receptors. Broad expression of these PRRs in the CNS and the release of DAMPs in and around sites of injury suggest an important role for these receptor families in mediating post-injury inflammation. Considerable data now show that PRRs are among the first responders to CNS injury and activation of these receptors on microglia, neurons, and astrocytes triggers an innate immune response in the brain and spinal cord. Here we discuss how the various PRR families are activated and can influence injury and repair processes following CNS injury.
Keywords: Inflammasome; NOD-like receptors; Neuroinflammation; Pattern recognition receptors; Spinal cord injury; Toll-like receptors.
Copyright © 2014. Published by Elsevier Inc.
Figures



References
-
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009;29:534–544. - PubMed
-
- Adamczak SE. Molecular Recognition of DNA by the AIM2 Inflammasome Induces Neuronal Pyroptosis: Implications in Infection and Host Tissue Damage (Open Access Dissertations) 2012
-
- Ahmad F, Wang MY, Levi AD. Hypothermia for acute spinal cord injury — a review. World Neurosurg. 2013 - PubMed
-
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal–ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985a;42:1791–798. (Epub ahead of print) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

