Monocyte Expressed Macromolecular C1 and C1q Receptors as Molecular Sensors of Danger: Implications in SLE
- PMID: 25018754
- PMCID: PMC4071343
- DOI: 10.3389/fimmu.2014.00278
Monocyte Expressed Macromolecular C1 and C1q Receptors as Molecular Sensors of Danger: Implications in SLE
Abstract
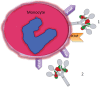
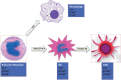
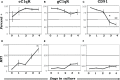
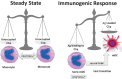
The ability of circulating blood monocytes to express C1q receptors (cC1qR and gC1qR) as well as to synthesize and secrete the classical pathway proteins C1q, C1r, and C1s and their regulator, C1-INH is very well established. What is intriguing, however, is that, in addition to secretion of the individual C1 proteins monocytes are also able to display macromolecular C1 on their surface in a manner that is stable and functional. The cell surface C1 complex is presumably formed by a Ca(2+)-dependent association of the C1r2⋅C1s2 tetramer to C1q, which in turn is anchored via a membrane-binding domain located in the N-terminus of its A-chain as shown previously. Monocytes, which circulate in the blood for 1-3 days before they move into tissues throughout the body, not only serve as precursors of macrophages and dendritic cells (DCs), but also fulfill three main functions in the immune system: phagocytosis, antigen presentation, and cytokine production. Since the globular heads of C1q within the membrane associated C1 are displayed outwardly, we hypothesize that their main function - especially in circulating monocytes - is to recognize and capture circulating immune complexes or pathogen-associated molecular patterns in the blood. This in turn may give crucial signal, which drives the monocytes to migrate into tissues, differentiate into macrophages or DCs, and initiate the process of antigen elimination. Unoccupied C1q on the other hand may serve to keep monocytes in a pre-dendritic phenotype by silencing key molecular players thus ensuring that unwarranted DC-driven immune response does not occur. In this paper, we will discuss the role of monocyte/DC-associated C1q receptors, macromolecular C1 as well as secreted C1q in both innate and acquired immune responses.
Keywords: C1q and C1q receptors; C1q in SLE; C1q in autoimmunity; DC and C1; c1q; monocyte C1.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

