Aberrant Proliferation of Differentiating Alveolar Cells Induces Hyperplasia in Resting Mammary Glands of SV40-TAg Transgenic Mice
- PMID: 25019062
- PMCID: PMC4071642
- DOI: 10.3389/fonc.2014.00168
Aberrant Proliferation of Differentiating Alveolar Cells Induces Hyperplasia in Resting Mammary Glands of SV40-TAg Transgenic Mice
Abstract
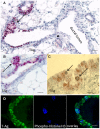
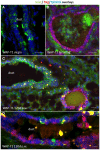
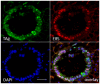
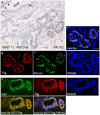
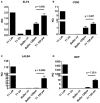
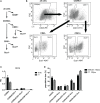
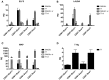
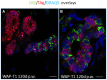
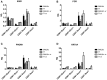
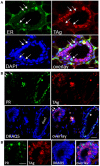
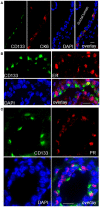
WAP-T1 transgenic mice express SV40-TAg under control of the whey acidic protein (WAP) promoter, which directs activity of this strong viral oncogene to luminal cells of the mammary gland. Resting uniparous WAP-T1 glands develop hyperplasia composed of TAg positive cells prior to appearance of advanced tumor stages. We show that cells in hyperplasia display markers of alveolar differentiation, suggesting that TAg targets differentiating cells of the alveolar compartment. The glands show significant expression of Elf5 and milk genes (Lalba, Csn2, and Wap). TAg expressing cells largely co-stain with antibodies to Elf5, lack the epithelial marker Sca1, and are hormone receptor negative. High expression levels of Elf5 but not of milk genes are also seen in resting glands of normal BALB/c mice. This indicates that expression of Elf5 in resting WAP-T1 glands is not specifically induced by TAg. CK6a positive luminal cells lack TAg. These cells co-express the markers prominin-1, CK6a, and Sca1, and are positive for hormone receptors. These hormone sensitive cells localize to ducts and seem not to be targeted by TAg. Despite reaching an advanced stage in alveolar differentiation, the cells in hyperplasia do not exit the cell cycle. Thus, expression of TAg in conjunction with regular morphogenetic processes of alveologenesis seem to provide the basis for a hormone independent, unscheduled proliferation of differentiating cells in resting glands of WAP-T1 transgenic mice, leading to the formation of hyperplastic lesions.
Keywords: SV40-TAg; alveologenesis; hyperplasia; mammary gland; tumorigenesis.
Figures
References
-
- Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol (2008) 32(4):513–23 10.1097/PAS.0b013e318161d1a5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

