Evaluation of the induction of immune memory following infant immunisation with serogroup C Neisseria meningitidis conjugate vaccines--exploratory analyses within a randomised controlled trial
- PMID: 25020050
- PMCID: PMC4096514
- DOI: 10.1371/journal.pone.0101672
Evaluation of the induction of immune memory following infant immunisation with serogroup C Neisseria meningitidis conjugate vaccines--exploratory analyses within a randomised controlled trial
Abstract
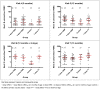
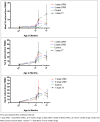
Aim: We measured meningococcal serogroup C (MenC)-specific memory B-cell responses in infants by Enzyme-Linked Immunospot (ELISpot) following different MenC conjugate vaccine schedules to investigate the impact of priming on immune memory.
Methods: Infants aged 2 months were randomised to receive 1 or 2 doses of MenC-CRM197 at 3 or 3 and 4 months, 1 dose of MenC-TT at 3 months, or no primary MenC doses. All children received a Haemophilus influenzae type b (Hib)-MenC booster at 12 months. Blood was drawn at 5, 12, 12 months +6 days and 13 months of age.
Results: Results were available for 110, 103, 76 and 44 children from each group respectively. Following primary immunisations, and prior to the 12-month booster, there were no significant differences between 1- or 2-dose primed children in the number of MenC memory B-cells detected. One month following the booster, children primed with 1 dose MenC-TT had more memory B-cells than children primed with either 1-dose (p = 0.001) or 2-dose (p<0.0001) MenC-CRM197. There were no differences in MenC memory B-cells detected in children who received 1 or 2 doses of MenC-CRM197 in infancy and un-primed children.
Conclusions: MenC-specific memory B-cell production may be more dependent on the type of primary vaccine used than the number of doses administered. Although the mechanistic differences between MenC-CRM197 and MenC-TT priming are unclear, it is possible that structural differences, including the carrier proteins, may underlie differential interactions with B- and T-cell populations, and thus different effects on various memory B-cell subsets. A MenC-TT/Hib-MenC-TT combination for priming/boosting may offer an advantage in inducing more persistent antibody.
Trial registration: EU Clinical Trials Register 2009-016579-31 ClinicalTrials.gov NCT01129518.
Conflict of interest statement
Figures



Similar articles
-
Is a single infant priming dose of meningococcal serogroup C conjugate vaccine in the United Kingdom sufficient?Hum Vaccin Immunother. 2015;11(6):1501-6. doi: 10.1080/21645515.2015.1019189. Hum Vaccin Immunother. 2015. PMID: 25912095 Free PMC article. Review.
-
Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial.BMJ. 2015 Apr 1;350:h1554. doi: 10.1136/bmj.h1554. BMJ. 2015. PMID: 25832102 Free PMC article. Clinical Trial.
-
Immunogenicity, reactogenicity, and immune memory after primary vaccination with a novel Haemophilus influenzae-Neisseria meningitidis serogroup C conjugate vaccine.Clin Vaccine Immunol. 2007 Apr;14(4):426-34. doi: 10.1128/CVI.00377-06. Epub 2007 Feb 7. Clin Vaccine Immunol. 2007. PMID: 17287313 Free PMC article. Clinical Trial.
-
Use of a booster dose of capsular group C meningococcal glycoconjugate vaccine to demonstrate immunologic memory in children primed with one or two vaccine doses in infancy.Vaccine. 2016 Dec 7;34(50):6350-6357. doi: 10.1016/j.vaccine.2016.10.038. Epub 2016 Oct 28. Vaccine. 2016. PMID: 28029540 Clinical Trial.
-
Update on the use of meningococcal serogroup C CRM₁₉₇-conjugate vaccine (Meningitec) against meningitis.Expert Rev Vaccines. 2016;15(1):9-29. doi: 10.1586/14760584.2016.1115726. Epub 2015 Nov 26. Expert Rev Vaccines. 2016. PMID: 26560735 Review.
Cited by
-
Is a single infant priming dose of meningococcal serogroup C conjugate vaccine in the United Kingdom sufficient?Hum Vaccin Immunother. 2015;11(6):1501-6. doi: 10.1080/21645515.2015.1019189. Hum Vaccin Immunother. 2015. PMID: 25912095 Free PMC article. Review.
-
Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial.BMJ. 2015 Apr 1;350:h1554. doi: 10.1136/bmj.h1554. BMJ. 2015. PMID: 25832102 Free PMC article. Clinical Trial.
-
Human Infant Memory B Cell and CD4+ T Cell Responses to HibMenCY-TT Glyco-Conjugate Vaccine.PLoS One. 2015 Jul 20;10(7):e0133126. doi: 10.1371/journal.pone.0133126. eCollection 2015. PLoS One. 2015. PMID: 26191794 Free PMC article. Clinical Trial.
-
Human B Cell Responses to Dominant and Subdominant Antigens Induced by a Meningococcal Outer Membrane Vesicle Vaccine in a Phase I Trial.mSphere. 2022 Feb 23;7(1):e0067421. doi: 10.1128/msphere.00674-21. Epub 2022 Jan 26. mSphere. 2022. PMID: 35080470 Free PMC article. Clinical Trial.
References
-
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E (2010) Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 17: 840–847. - PMC - PubMed
-
- Borrow R, Balmer P, Miller E (2005) Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 23: 2222–2227. - PubMed
-
- Maiden MC, Stuart JM (2002) Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359: 1829–1831. - PubMed
-
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME (2004) Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364: 365–367. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

