Bidirectional modulation of deep cerebellar nuclear cells revealed by optogenetic manipulation of inhibitory inputs from Purkinje cells
- PMID: 25020121
- PMCID: PMC5862060
- DOI: 10.1016/j.neuroscience.2014.07.006
Bidirectional modulation of deep cerebellar nuclear cells revealed by optogenetic manipulation of inhibitory inputs from Purkinje cells
Abstract
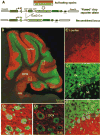
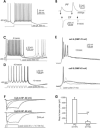
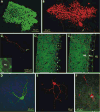
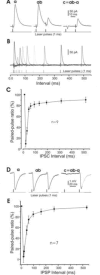
In the mammalian cerebellum, deep cerebellar nuclear (DCN) cells convey all information from cortical Purkinje cells (PCs) to premotor nuclei and other brain regions. However, how DCN cells integrate inhibitory input from PCs with excitatory inputs from other sources has been difficult to assess, in part due to the large spatial separation between cortical PCs and their target cells in the nuclei. To circumvent this problem we have used a Cre-mediated genetic approach to generate mice in which channelrhodopsin-2 (ChR2), fused with a fluorescent reporter, is selectively expressed by GABAergic neurons, including PCs. In recordings from brain slice preparations from this model, mammalian PCs can be robustly depolarized and discharged by brief photostimulation. In recordings of postsynaptic DCN cells, photostimulation of PC axons induces a strong inhibition that resembles these cells' responses to focal electrical stimulation, but without a requirement for the glutamate receptor blockers typically applied in such experiments. In this optogenetic model, laser pulses as brief as 1 ms can reliably induce an inhibition that shuts down the spontaneous spiking of a DCN cell for ∼50 ms. If bursts of such brief light pulses are delivered, a fixed pattern of bistable bursting emerges. If these pulses are delivered continuously to a spontaneously bistable cell, the immediate response to such photostimulation is inhibitory in the cell's depolarized state and excitatory when the membrane has repolarized; a less regular burst pattern then persists after stimulation has been terminated. These results indicate that the spiking activity of DCN cells can be bidirectionally modulated by the optically activated synaptic inhibition of cortical PCs.
Keywords: Purkinje cell; deep nuclear cell; optogenetic model; photostimulation; synaptic inhibition.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures





References
-
- Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21(4):827–835. - PubMed
-
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82(4):1697–1709. - PubMed
-
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

